Congenital bilateral permanent hearing loss has a major impact on speech and language development.1 Estimates of the prevalence of such hearing loss range from 0.53–1.50 per 1000 live births2-6 (Appendix 1). Studies have shown that children with hearing loss who receive early intervention have better language skills than those with later intervention.1,7 The critical age by which intervention should commence may be as early as six months.1 However, diagnosis is often delayed. In 1997, in Western Australia, the median age for fitting of hearing aids for congenital hearing loss was 25 months.8
Two approaches to newborn hearing screening have been used — targeted screening of babies with risk factors using criteria such as those of the Joint Committee on Infant Hearing 20009 (Appendix 2), or universal screening of all newborns. As only about 60% of children with congenital hearing loss can be identified by using high-risk criteria,2,3,6 universal hearing screening has been recommended in the United States9 and the United Kingdom.10
We report the preliminary findings of a pilot program of newborn hearing screening to detect congenital bilateral permanent hearing loss at five maternity hospitals in Perth, Western Australia. The program commenced in 2000 and our report includes results in babies born until 30 June 2001.
All liveborn infants at the screening hospitals were eligible for screening, which was offered to well babies generally on the day after delivery. Babies admitted to a Level 2 or 3 nursery were screened when the baby was at least 34 weeks' gestation. If possible, the parents of babies who were discharged before screening were given an invitation by the visiting midwife to return for an outpatient screen.
We developed a combined data and consent form, and recorded for all babies (whether screened or not) delivery site, date and time of birth, and sex. If the baby was not screened, the reason for not screening was recorded. For screened babies, demographic information, birthweight, gestational age and screening details were collected.
We used a combined-technology screen to increase specificity and reduce costs. The well-baby screening flow diagram and a description of the two screening instruments used are given in Box 1. Babies with a significant family history of hearing loss who passed the hearing screen were referred for audiological assessment at age seven months.
After the hearing screen parents were given a results sheet which explained the results and reinforced the need for ongoing childhood hearing surveillance. The results of the screen were recorded in the baby's hospital records and personal health record.
There were 13 214 eligible babies. Of these, 12 708 (96.2%) were screened. The proportion of babies screened increased with time from 93.5% of those born before July 2000 to 97.1% of those born in the first six months of 2001 (Box 2). About 18% of babies required Level 2 or 3 nursery care.
Of the babies screened at Princess Margaret Hospital from the start of its newborn screening program in June 2001, only those transferred from a hospital where screening was available were included in the data analysis.
About 80% of all babies screened had measurement of transient evoked otoacoustic emissions (TEOAE) only, while about 16% also had automated auditory brainstem responses (AABR) measured (Box 1). Most of the 4% of babies screened using only AABR were in Level 2 or 3 nurseries.
Early discharge (309; 2.3%) was the main reason that babies were not screened (Box 2). By 2001, more women took up the offer of an outpatient screen for their infant, and the proportion who missed screening decreased to 1.8%. Of the 129 (1.0%) babies not screened because of transfer to another hospital, 69 were screened as part of a high-risk screening program in the Level 3 nursery at Princess Margaret Hospital. All babies in the high-risk program were screened by an audiologist before hospital discharge. (Information was available on the outcome for these 69 babies only. They are included in the calculation of prevalence of hearing loss in the cohort, but not in the evaluation of the screening process.)
Box 2 shows the results of the initial screening. The bilateral "pass" rate increased over time. A total of 351 babies failed the screen in one or both ears. In the first weeks of the program, an AABR instrument was not available, so babies were screened using only TEOAE, and follow-up was only offered to babies who failed the screen in both ears. Thus, 40 babies who failed the screen in one ear were not offered follow-up. Another 10 babies were referred directly for audiological assessment due to either repeated fail responses to hearing screening while in a Level 2 or 3 nursery, or a structural problem affecting the ears. Of the remaining 301 babies who were offered follow-up, 271 (90%) returned and 30 (10%) babies were lost to follow-up due to multiple missed appointments or parental refusal. When families did not attend the follow-up appointment, their child health nurse was notified and asked to encourage the family to arrange a repeat screen, or, failing this, to monitor the baby's hearing.
Of the babies referred after follow-up screening, five were diagnosed with bilateral hearing loss by the age of three months and three had unilateral loss.
Of the 69 babies screened as part of the high-risk program at Princess Margaret Hospital, four were diagnosed with bilateral hearing loss.
Thus, there were nine cases of permanent bilateral hearing loss (> 35 dB in the better ear) diagnosed in this cohort of babies, making the prevalence of bilateral permanent hearing loss 0.68 per 1000 eligible babies (95% CI, 0.31–1.28). Eight of these nine babies had at least one hearing-loss risk factor9 (Appendix 2), including five who had received Level 2 or 3 nursery care. The rate of bilateral permanent hearing loss in well babies was 0.37 per 1000 (95% CI, 0.10–0.92). Two of the four well babies had a family history of hearing loss.
Of the nine babies with permanent bilateral hearing loss, six were fitted with hearing aids by the age of six months, and one was fitted with aids at 19 months because of parental delays. One baby who received long-term Level 3 nursery care is still in hospital and his condition has precluded fitting a hearing aid, and one baby has died.
During the first 17 months of this program, the prevalence of congenital bilateral hearing loss was 0.68 per 1000, with eight out of nine affected babies having a risk factor for hearing loss. A summary of other hearing screening programs is given in Box 3. However, the different definitions of hearing loss used make comparison between studies difficult.
Our program meets the recommendations of the Joint Committee on Infant Hearing9 for process quality indicators, with 96.2% screening uptake, 90% return for follow-up and 0.18% referral for audiological assessment. However, our study has revealed a number of weaknesses: (i) the confidence intervals for the prevalence of bilateral hearing loss were wide; (ii) our cohort is not representative of all Western Australian births; (iii) the screening instrument we used has not been well validated in the medical literature; and (iv) we had limited time for diagnosis of hearing loss in babies who passed the hearing screening ("false negatives").
Despite our large sample size, the 95% CIs for the prevalence of bilateral congenital hearing loss are wide (0.31–1.28). However, most other screening programs have equally wide CIs (Box 3). There is a strong case to continue screening, so that, with a larger sample, there will a more precise estimate of prevalence.
In our cohort of babies those requiring special care are over-represented, as screening is offered at all delivery sites with Level 2 or 3 nursery facilities. Since these babies are more likely to have hearing loss, the prevalence we calculated is likely be higher than in the State as a whole. There are few reports of screening programs covering the whole population of a defined geographical area.6,27,28
We have detected a prevalence of bilateral congenital hearing loss that is at the lower end of those found in population-based studies (Appendix 1). We found that eight of the nine babies with bilateral hearing loss had a risk factor for hearing loss, which is a higher proportion than others have reported.2,3,6 Little is known about the prevalence and aetiology of newborn hearing loss in Western Australia. According to the report of the Birth Defects Registry of Western Australia, 1980–2000,31 the prevalence of congenital deafness in Western Australia was 0.7 per 1000 births between 1980 and 1994, although under-reporting is suspected. By manipulating the data provided by Australian Hearing,32 the hearing-aid fitting rate for all causes of hearing loss greater than 30 dB in Western Australian children born in 1996 was 0.95 per 1000. However, from the information provided, the proportion of hearing loss thought to be congenital in origin cannot be calculated. To monitor both for false negative results and provide population-based prevalence data for Western Australia, a database is being set up to record all Western Australian children born in 1999 and later who are diagnosed with bilateral hearing loss before the age of five years.
Our program has been successful in screening a high percentage of eligible babies, with a low referral rate. However, the prevalence of bilateral permanent hearing loss detected is low (0.68/1000). Several aspects of the program are being evaluated and data about permanent hearing loss in Western Australian children will be obtained before deciding whether to extend the program to other hospitals.
1: Hearing loss screening instruments and flow diagram for newborn screening for well babies
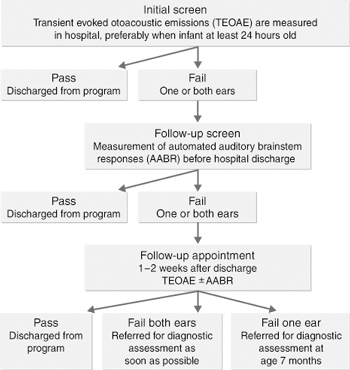
Measurement of transient evoked otoacoustic emissions (TEOAEs)
All well babies were screened using an Echocheck TEOAE hand-held screener (Otodynamics, Hatfield, UK), which involves placing a probe into the baby's outer ear. This test concentrates on the main speech frequency band range of 1.6–3.6 Hz. A "pass" response is based on detecting a non-linear TEOAE cross-correlated signal-to-noise ratio of at least 6 decibels (dB).
Automated auditory brainstem responses (AABR)
The detection of AABR is more specific, but the test takes more time and is more expensive than the TEOAE screen, requiring the placement of scalp electrodes. Until July 2000 the AABR instrument used was the Sabre system (SLE Ltd, South Croydon, UK). After July, the Algo 2e Color Newborn Hearing Screener (Natus Medical Inc, San Carlos, USA) was used. Auditory brainstem response is a modified electroencephalogram recording of brain activity in response to auditory stimuli presented in the form of brief clicks. By averaging techniques, the electrical potential can be detected and used to determine the hearing threshold. Both the Sabre and Algo 2e screening units use an automated detection of these responses and yield "pass"/"refer" criteria set at 35 dB normal hearing level scale.
Comments: Both the TEOAE and AABR screening instruments clearly display
a "pass" response, so no interpretation is required. The TEOAE screen has limited specificity when used in the first few days of life. It only measures outer hair-cell function in the cochlea. Babies with central hearing loss can have normal cochlear function and so will pass the TEOAE screen. As the group most at risk of central hearing loss are babies who have received Level 3 care, most babies who have had long-term neonatal intensive care are screened initially using the AABR screen.
2: Uptake and results of the initial hearing screen and reasons initial screening was not completed
Number (%) babies born |
|||||||||||
Before 01/07/00 |
01/07/00–31/12/00 |
01/01/01–30/06/01 |
Total |
||||||||
Uptake of screening* |
|||||||||||
Eligible for screening |
2513 |
(100%) |
5325 |
(100%) |
5376 |
(100%) |
13 214 |
(100%) |
|||
Not screened |
165 |
(6.6%) |
186 |
(3.5%) |
155 |
(2.9%) |
506 |
(3.8%) |
|||
Discharged before screen |
107 |
(4.3%) |
103 |
(1.9%) |
99 |
(1.8%) |
309 |
(2.3%) |
|||
Transferred† |
37 |
(1.5%) |
53 |
(1.0%) |
39 |
(0.7%) |
129 |
(1.0%) |
|||
Refused screening |
14 |
(0.6%) |
23 |
(0.4%) |
14 |
(0.3%) |
51 |
(0.4%) |
|||
Missed screening‡ |
7 |
(0.3%) |
7 |
(0.1%) |
3 |
(0.1%) |
17 |
(0.1%) |
|||
Screen completed |
2348 |
(93.4%) |
5139 |
(96.5%) |
5221 |
(97.1%) |
12 708 |
(96.2%) |
|||
Results of screening§ |
|||||||||||
"Pass" both ears |
2200 |
(93.7%) |
5027 |
(97.8%) |
5130 |
(98.3%) |
12 357 |
(97.2%) |
|||
"Pass" one ear |
110 |
(4.7%) |
72 |
(1.4%) |
64 |
(1.2%) |
246 |
(1.9%) |
|||
"Fail" both ears |
38 |
(1.6%) |
40 |
(0.8%) |
27 |
(0.5%) |
105 |
(0.8%) |
|||
* Excludes babies who died before screening. † 69 of these babies, who were transferred to Princess Margaret Hospital, were screened as part of a high-risk hearing screening program at that hospital. ‡ Mostly babies who did not appear on the daily list of deliveries, and by the time the omission was discovered the local visiting midwife was no longer in contact with the family. § The denominator for these calculations was the number of babies screened. |
|||||||||||
3: Summary of the methods and results of published newborn hearing screening programs*
First author, country, years data collected |
Definition of bilateral hearing loss |
Population |
No. of cases/ population |
Rate per 1000 (95% CI) |
Proportion with risk factors |
Rate per 1000 well babies† (95% CI) |
|||||
Barsky-Firsker,13 USA, 1993–1995 |
Sensorineural, |
One hospital with NICU; |
46/15 749 |
2.92 |
2.07‡ |
||||||
Chapchap,14 |
All, moderate |
One hospital with NICU; |
7/4 196 |
1.67 |
71% |
||||||
Clemens,15 |
Permanent, |
One hospital; report of well baby screening only |
6/5 010 |
1.20 |
67% |
1.20 |
|||||
Clemens,16 |
Permanent, |
One hospital; report of well baby screening only |
2/3 142 |
0.64 |
38%‡ |
0.64 |
|||||
Permanent, |
Eight hospitals, all with NICUs; 2435–5474 births a year |
49/43 311 |
1.13 |
80% |
|||||||
Finitzo,19 |
Permanent, |
Eleven sites; < 300–4540 births a year; four NICUs |
32/17 105 |
1.87 |
47%‡ |
1.26‡ |
|||||
Isaacson,20 |
≥ 25 dB |
One hospital with NICU; |
6/2 031 |
2.95 |
|||||||
Kanne,21 |
Permanent |
One hospital with NICU; |
1/2 289 |
0.44 |
100% |
||||||
Lim,22 |
> 35 dB§ |
46 sites in 11 States; |
57/66 292 |
0.86 |
40%‡ |
||||||
Mason,23 |
Permanent, |
One hospital with NICU; |
15/10 372 |
1.45 |
0.89 |
||||||
Mehl,24 |
Sensorineural |
26/52 hospitals (40–3500 births a year); 60% of State's births |
75/41 796 |
1.79 |
50%ঠ|
||||||
Sergi,25 |
|
One hospital |
Well babies |
1/5 650 |
0.18 |
0.18 |
|||||
|
|
NICU, no risk |
3/749 |
4.01 |
|||||||
|
|
At risk |
14/118 |
118.64 |
|||||||
Stewart,26 |
Sensorineural, |
Five sites; one NICU; two Level 2 nurseries |
21/11 711 |
1.79 |
|||||||
Watkin,27 |
Permanent, |
District; 3500 births a year |
23/11 606 |
1.98 |
|||||||
Watkin,28 |
Permanent, |
District; 3500 births a year |
34/25 199 |
1.35 |
|||||||
Wessex Group,29 |
Permanent, |
Four hospitals; four SCN; 3300–5600 births a year |
27/25 609** |
1.05 |
74% |
0.84†† |
|||||
White,30 |
Sensorineural, |
One hospital with NICU |
6/1 850 |
3.24 |
83% |
1.29 |
|||||
* Some values have been calculated from the data provided in the studies. Where possible, all cases of temporary or unilateral hearing loss have been excluded. |
|||||||||||
Appendix 1: Summary of population-based studies* on the prevalence of congenital, permanent bilateral hearing loss
Study |
Definition of bilateral hearing loss |
Characteristics of population |
No. of cases/ population |
Rate per 1000 (95% CI) |
Proportion with risk |
Rate per 1000 well babies† (95% CI) |
|||||
Fortnum and Davis2 1997 |
≥ 40 dB, permanent |
Born 1985–1990, living in Trent region, UK, in 1994–1995 |
487/366 480 |
1.33 |
|||||||
≥ 40 dB, congenital |
16% of hearing loss thought to be acquired later |
409/366 480 |
1.12 |
58.9% |
0.84‡ |
||||||
Parving3 1993 |
≥ 25 dB, requiring hearing aid |
Born 1980–1990, living in Copenhagen city or county in 1992. Those with hearing loss had a hearing aid fitted by January 1992 |
181/95 912 |
1.89 |
|||||||
≥ 25 dB, congenital/ early acquired |
20% of hearing loss thought to be acquired later |
144/95 912 |
1.50 |
59.7% |
|||||||
Van Naarden |
≥ 40 dB, permanent |
Born 1981–1990, living in five Atlanta counties, USA, in 1991–1993 |
862/790 200§ |
1.09 |
|||||||
|
≥ 40 dB, congenital (sensorineural cases with no postneonatal event recorded) |
50% of sensorineural hearing loss thought to be acquired later |
173/324 327¶ |
0.53 |
|||||||
Vartiainen et al5 1997 |
> 25 dB, sensorineural |
Born 1974–1987 in Kuopio region, Finland |
98/46 240 |
2.12 |
|
||||||
|
> 25 dB, congenital |
34% of hearing loss > 25 dB thought to be acquired later |
65/46 240 |
1.41 |
|||||||
|
> 40 dB, sensorineural |
52/46 240 |
1.12 |
||||||||
> 40 dB, congenital |
17% of hearing loss > 40 dB thought to be acquired later |
41/46 240 |
0.89 |
||||||||
Vohr et al6 1998 |
> 30 dB, permanent hearing loss, all presumed congenital |
Born 1993–1996, Rhode Island, USA, population-based screening program, no mention of later acquired hearing loss |
79/53 121 |
1.49 |
60%**†† |
1.27** |
|||||
* Some values have been calculated from the data provided in the reports. |
|||||||||||
Appendix 2: Indicators associated with sensorineural and/or conductive hearing loss for use
in neonates when universal screening is not available*
An illness or condition requiring admission of 48 hours or more to a neonatal intensive care unit
Stigmata or other findings associated with a syndrome known to include a sensorineural and/or conductive hearing loss.
Family history of permanent childhood sensorineural hearing loss.
Craniofacial anomalies, including those with morphological abnormalities of the pinna and ear canal.
In-utero infection, such as cytomegalovirus, herpes and toxoplasmosis, or rubella.
* Joint Committee on Infant Hearing.9
Received 19 September 2001, accepted 12 April 2002
- Helen D Bailey1
- Carol Bower2
- Jay Krishnaswamy3
- Harvey L Coates4
- 1 Centre for Child Health Research, University of Western Australia, TVW Telethon Institute for Child Health Research, West Perth, WA.
- 2 Princess Margaret Hospital for Children, Perth, WA.
We acknowledge the support of the babies' parents; the hospital staff; the hearing screeners (J MacLean, F Smith, J Zach, R Clack, R Jaganathan, M Gualda-Barr, A McHarrie, M Biggs, L Hooper, L Hollywood and S Yong); the data entry clerks (A Freemantle and R Silburn); and Professor Fiona Stanley AC, and members of the newborn hearing screening steering committee: C Bull, P Howes (Australian Hearing Services); M Bulsara (Department of Public Health UWA); Dr N French (King Edward Memorial Hospital); Dr M Gibson, V Verma (State Child Development Centre); P Higginbotham (the Speech and Hearing Centre); John Richards (WA Institute for Deaf Education); S Weeks (Disability Services Commission).
The program is funded by the Department of Health in Western Australia, King Edward Memorial and Princess Margaret hospitals, and the Garnett-Passe and Rodney Williams Memorial Foundation. C Bower is funded by NHMRC Fellowship number 172303.
None identified. The Department of Health, Western Australia, and King Edward Memorial and Princess Margaret hospitals were involved in the planning of the pilot program. The funding bodies had no input into the analysis or interpretation of the data, the writing of the article or the decision to submit for publication.
- 1. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and later-identified children with hearing loss. Pediatrics 1998; 102: 1161-1171.
- 2. Fortnum H, Davis A. Epidemiology of permanent childhood hearing impairment in Trent Region, 1985-1993. Br J Audiol 1997; 31: 409-446.
- 3. Parving A. Congenital hearing disability — epidemiology and identification: a comparison between two health authority districts. Int J Pediatr Otorhinolaryngol 1993; 27: 29-46.
- 4. Van Naarden K, Decoufle P, Caldwell K. Prevalence and characteristics of children with serious hearing impairment in metropolitan Atlanta, 1991-1993. Pediatrics 1999; 103: 570-575.
- 5. Vartiainen E, Kemppinen P, Karjalainen S. Prevalence and etiology of bilateral sensorineural hearing impairment in a Finnish childhood population. Int J Pediatr Otorhinolaryngol 1997; 41: 175-185.
- 6. Vohr BR, Carty LM, Moore PE, Letourneau K. The Rhode Island Hearing Assessment Program: experience with statewide hearing screening (1993-1996). J Pediatr 1998; 133: 353-357.
- 7. Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics 2000; 106: E43.
- 8. Eastough NJ, Gibson MJ. Western Australian child and youth health goals and targets: a progress report with national comparisions. Perth: Health Information Centre, Health Department of Western Australia, 1999.
- 9. Joint Committee on Infant Hearing. Position statement: principles and guidelines for early hearing detection and intervention programs. Am J Audiol 2000; 9: 9-29.
- 10. Davis A, Bamford J, Wilson I, et al. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technol Assess 1997; 1(10).
- 11. Filemaker Pro 5.0 [computer program], version 3 Santa Clara, Calif: FileMaker, Inc, 2000.
- 12. Ury HK, Wiggins AD. Another shortcut method for calculating the confidence interval of a Poisson variable (or of a standardized mortality ratio). Am J Epidemiol 1985; 122: 197-198.
- 13. Barsky-Firsker L, Sun S. Universal newborn hearing screenings: a three-year experience. Pediatrics 1997; 99: E4.
- 14. Chapchap MJ, Segre CM. Universal newborn hearing screening and transient evoked otoacoustic emission: new concepts in Brazil. Scand Audiol Suppl 2001; 53: 33-36.
- 15. Clemens CJ, Davis SA, Bailey AR. The false-positive in universal newborn hearing screening. Pediatrics 2000; 106: E7.
- 16. Clemens CJ, Davis SA. Minimizing false-positives in universal newborn hearing screening: a simple solution. Pediatrics 2001; 107: E29.
- 17. Dalzell L, Orlando M, MacDonald M, et al. The New York State universal newborn hearing screening demonstration project: ages of hearing loss identification, hearing aid fitting, and enrollment in early intervention. Ear Hear 2000; 21: 118-130.
- 18. Prieve BA, Stevens F. The New York State universal newborn hearing screening demonstration project: introduction and overview [see comments]. Ear Hear 2000; 21: 85-91.
- 19. Finitzo T, Albright K, O'Neal J. The newborn with hearing loss: detection in the nursery. Pediatrics 1998; 102: 1452-1460.
- 20. Isaacson G. Universal newborn hearing screening in an inner-city, managed care environment. Laryngoscope 2000; 110: 881-894.
- 21. Kanne TJ, Schaefer L, Perkins JA. Potential pitfalls of initiating a newborn hearing screening program. Arch Otolaryngol Head Neck Surg 1999; 125: 28-32.
- 22. Lim G, Fortaleza K. Overcoming challenges in newborn hearing screening. J Perinatol 2000; 20 (8 Pt 2): S138-S142.
- 23. Mason JA, Herrmann KR. Universal infant hearing screening by automated auditory brainstem response measurement [see comments]. Pediatrics 1998; 101: 221-228.
- 24. Mehl AL, Thomson V. Newborn hearing screening: the great omission. Pediatrics 1998; 101: E4.
- 25. Sergi P, Pastorino G, Ravazzani P, et al. A hospital based universal neonatal hearing screening programme using click-evoked otoacoustic emissions. Scand Audiol Suppl 2001; 52: 18-20
- 26. Stewart DL, Mehl A, Hall JW, 3rd, et al. Universal newborn hearing screening with automated auditory brainstem response: a multisite investigation. J Perinatol 2000; 20 (8 Pt 2): S128-S131.
- 27. Watkin PM. Neonatal otoacoustic emission screening and the identification of deafness [see comments]. Arch Dis Child Fetal Neonatal Ed 1996; 74: F16-F25.
- 28. Watkin PM, Baldwin M. Confirmation of deafness in infancy. Arch Dis Child 1999; 81: 380-389.
- 29. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. Wessex Universal Neonatal Hearing Screening Trial Group [see comments]. Lancet 1998; 352: 1957-1964.
- 30. White KR, Vohr BR, Maxon AB, et al. Screening all newborns for hearing loss using transient evoked otoacoustic emissions. Int J Pediatr Otorhinolaryngol 1994; 29: 203-217.
- 31. Bower C, Rudy E, Ryan A, Cosgrove P. Report of the Birth Defects Registry of Western Australia, 1980-2000. Subiaco: King Edward Memorial Hospital Centre for Women's Health, 2001. (Report No. 8.)
- 32. Australian Hearing. Details and aetiology of persons under the age of 17 years with a hearing impairment who have been fitted with a hearing aid, 31 March 2001: Australian Hearing, 2001. (Audiology Circular: 2001/6.)





Abstract
Aim: To report the preliminary findings of a pilot program to screen newborn babies for congenital bilateral permanent hearing loss.
Setting: The five largest maternity hospitals in Perth, Western Australia. Screening was gradually introduced over seven months from February to August 2000.
Participants: All babies born at these hospitals after the introduction of hearing screening until 30 June 2001.
Methods: One or both of two automated screening devices were used: one measuring transient evoked otoacoustic emissions (TEOAE) and the other automated auditory brainstem responses (AABR). If a "pass" was not obtained in both ears, screening was repeated. All babies who did not obtain a pass in either ear at follow-up were referred for audiological assessment.
Main outcome measures: Prevalence of permanent bilateral hearing loss.
Results: Of 13 214 eligible babies, 12 708 (96.2%) received screening. The main reason for missing screening was early hospital discharge (309; 2.3%). Of the screened babies, 99% had a pass response in both ears at either the initial or follow-up screen. Twenty-three babies were referred for audiological assessment, and nine were diagnosed with bilateral permanent hearing loss (0.68/1000; 95% CI, 0.31–1.28).
Conclusions: Despite our program meeting process quality indicators, our detection rate was low. Before extending the program to smaller hospitals, we need to validate our screening instruments and put in place a system to monitor false negative results.