On average, patients with end-stage kidney disease (ESKD) live 10 years longer if they receive a kidney transplant than if they remain on dialysis.1 Falling per-capita deceased organ-donor numbers and longer kidney transplant waiting lists in Australia2 have increased the emphasis on live kidney donation for these patients. Based on the prevalence of ABO blood groups and of human leukocyte antigen (HLA) sensitisation of recipients, it is estimated that approximately 30% of willing and otherwise appropriate kidney donor–recipient pairs are biologically incompatible and do not proceed to living donor transplantation. Paired kidney exchange (PKE) is a relatively new strategy that helps these patients find suitable donors when their willing, living donors are unsuitable for them because of blood group or HLA incompatibility.3
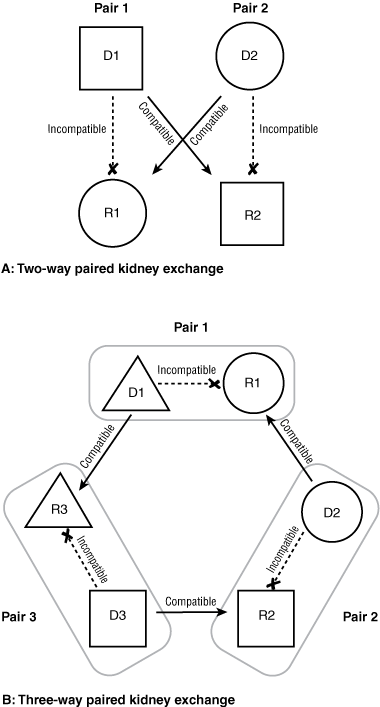
As shown in Box 1, A, a paired exchange can happen when a living donor (D1) and the intended recipient (R1), unable to proceed with the donation because of incompatible blood or tissue type, are matched with another registered incompatible pair (D2 and R2) by an exchange of donors between the pairs, making two compatible living donor transplants possible. Successful PKE programs operate in the Netherlands, South Korea, the United Kingdom and United States. In Australia, PKE is an emerging practice, and the first PKE transplant took place in Western Australia in October 2007. A high-level working group of clinicians and government officials has recently made recommendations to Australian federal and state governments on how a national PKE program could be successfully implemented.
In conventional PKE donations, two donor–recipient pairs surmount each other’s incompatibility problem by simply exchanging donors (Box 1, A).3,4 PKE donations can be arranged involving three or more pairs (Box 1, B) — a complex procedure that, for optimal matching, relies on sophisticated computer software.4-6 In conventional PKE donations, the only way to ensure that both recipients in a paired exchange receive their grafts (ie, that neither donor withdraws from the exchange agreement) is to perform the two transplantations simultaneously. Such conventional two-way or three-way PKE donations require patients with incompatible living donors to be matched to pairs with reciprocal incompatibilities. The probability of finding a suitable donor–recipient pair for an exchange is greatly influenced by the pool size. Even in a successful large national PKE program, only around 50% of incompatible pairs find a match and undergo transplantation, primarily because of the blood group imbalance in the pool of incompatible pairs.4 Because most blood group-O donors can give directly to their intended recipients, O donors will enter the PKE pool only if they have a positive crossmatch with their recipient, indicating the recipient is sensitised to the donor. Therefore, blood group-O donors must be preferentially matched with blood group-O recipients4 to give these recipients the fairest chance of receiving a transplant. With a pool size of around 100 donor–recipient pairs, the chance of a match is around 60% for ABO-incompatible or sensitised non-O recipients and 15% for ABO-incompatible O recipients.4
Altruistic, or non-directed, donors are becoming increasingly available.7 Allocating these donors to the PKE program can result in a domino effect and facilitate a number of transplants. For this reason, we suggest that altruistic donor kidneys should preferentially be allocated to the PKE program, given the increase in transplants this can bring. The potential of discriminating against highly sensitised patients on the deceased-donor list, who might otherwise receive a kidney from a non-directed donor, is averted by first attempting to match the donor against these patients and allocating the donor to the PKE program only if no match is found.
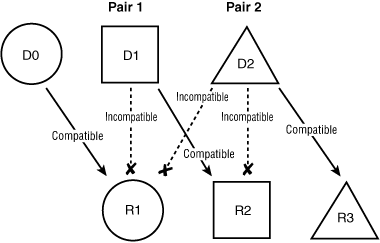
Altruistic donor chains, also called “domino paired donations”, are initiated by altruistic donors, and terminated by the last paired donor in the chain giving a kidney to an unpaired recipient on the deceased-donor waiting list.8 In Box 2, the altruistic donor (D0) is matched anonymously to a recipient (R1) who has a willing but incompatible living donor (D1). This donor gives a kidney to another recipient (R2) whose intended donor (D2) is incompatible. This last donor is matched with a recipient (R3) on the waiting list for deceased-donor organs. When an altruistic donor initiates a chain of transplants, no other donor ever gives a kidney until their own co-registered recipient has received a transplant. Thus, although the withdrawal of a donor in the middle of a chain would still constitute a breach of commitment, it would not harm the remaining pairs in the chain, allowing the possibility of non-simultaneous transplants. Thus, when paired donations are initiated by an altruistic donor, they can lead to multiple paired transplants and they do not necessarily have to be performed simultaneously.
Selection of donor–recipient pairs within the PKE pool should maximise the potential number of suitable recipient pairs, taking into account the match probability of any recipient having a suitable (ie, blood group-compatible, crossmatch-negative) donor.4,6 Priority should be given to ABO-identical pairs to maximise the likelihood of O recipients receiving a kidney and to help overcome their disadvantage. An exception to this rule can be made in cases of highly sensitised non-O recipients who have a very low chance of finding an acceptable crossmatch-negative donor. Furthermore, match probability needs to take account of recipient HLA antibody profiles and the frequency with which these are likely to react with those within the donor pool. The Dutch PKE program uses these two principles and has proven to be the single most efficient PKE program.4 Further prioritisation can take place according to donor and recipient location, donor–recipient age difference, prior cytomegalovirus exposure and more. However, increasing the number of additional discriminators in the matching process increases the likelihood of not finding crossmatch-negative donors for the incompatible pairs.
Blood group-incompatible transplantation has become an acceptable procedure in selected individuals and in particular provides a transplant option for blood group-O recipients, who have to wait the longest for a deceased-donor kidney and are less likely to have a blood group-compatible living donor available. Several desensitisation protocols have been developed to overcome the blood group barrier, particularly since the introduction of antigen-specific immunoadsorption.9 A high initial titre of blood group antibodies has been shown to predict the failure of desensitisation protocols to reduce titre to a safe level for transplantation, and some centres avoid desensitisation in patients with pre-treatment titres exceeding 1:128.9 Thus, a PKE program for high-titre O recipients and an ABO-incompatible desensitisation program for low-titre O recipients are complementary and not competitive.
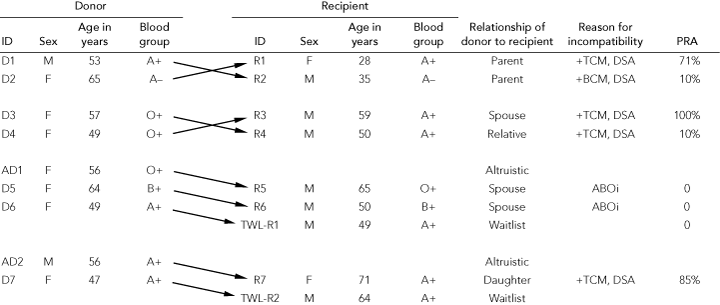
The PKE program in WA is based on a small single-centre pool with a transplant waiting list for deceased-donor kidneys of about 110 patients with ESKD. Using enrolled incompatible donor–recipient pairs and altruistic donors, the program successfully matched and transplanted kidneys into nine patients between October 2007 and November 2008 (Box 3). These transplants arose from three two-way simultaneous exchanges and one non-simultaneous three-way exchange. The latter was initiated by an altruistic donor, and transplants were performed on different days. Five recipients (three with antibody against > 70% of donors) had a positive crossmatch and donor-specific antibodies to their intended donor; two recipients had a donor blood group incompatibility; and the other two recipients were on the waiting list for deceased-donor organs and each received a kidney as the last link in an altruistic donor chain. All donors underwent laparoscopic nephrectomy without surgical complications. Each recipient had an uneventful course following transplantation; allograft function was immediate and remained good at 1 month.
Although PKE and altruistic donation programs are not the sole solution for the organ shortage in kidney transplantation, they have advantages over the more medically demanding programs, such as transplantation across the blood type or tissue barrier after desensitisation. These advantages include: a clinically prudent approach with a system of donor exchange that identifies recipient pairs who have no donor alloreactivity; cost saving by averting the need for plasmapheresis or immunoadsorption in the recipient; and avoidance of the hazard of a rejection episode associated with the reappearance of donor-specific antibodies by identifying exchange pairs whose recipients have no donor-specific antibodies before transplantation.10 Promotion of live kidney donation has been made easier by the introduction of the less invasive laparoscopic nephrectomy for donors, and by the achievement of excellent long-term donor outcomes.11 To achieve these outcomes, the criteria used for accepting donors must ensure their safety, particularly for those with risk factors for kidney disease, such as hypertension or glucose intolerance.
3 Characteristics of donors and recipients in the Western Australian paired kidney donation program, October 2007 to November 2008
- Paolo Ferrari1,2
- Claudia Woodroffe1
- Frank T Christiansen3,4
- 1 Department of Nephrology, Fremantle Hospital, Fremantle, WA.
- 2 School of Medicine and Pharmacology, University of Western Australia, Perth, WA.
- 3 Department of Clinical Immunology and Immunogenetics, PathWest, Royal Perth Hospital, Perth, WA.
- 4 School of Pathology and Laboratory Medicine, University of Western Australia, Perth, WA.
We would like to acknowledge the support and the advice in setting up the WA PKE program given by Marry de Klerk (Dutch PKE program, Rotterdam, The Netherlands), Inessa Kaplan (Johns Hopkins Hospital, Baltimore, Md, USA) and Ruthanne Hanto (New England PKE program, Newton, Mass, USA). We would also like to recognise the contribution of all nephrologists and surgeons, the dedication of all the transplant coordinators, the expertise of staff at the PathWest WA HLA laboratory and the support of the Office of Population Genomics in Perth.
None identified.
- 1. Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 2005; 16: 1859-1865.
- 2. Mathew T, Faull R, Snelling P. The shortage of kidneys for transplantation in Australia. Med J Aust 2005; 182: 204-205. <MJA full text>
- 3. Rapaport FT. The case for a living emotionally related international kidney donor exchange registry. Transplant Proc 1986; 18 (2 Suppl): 5S-9S.
- 4. de Klerk M, Witvliet MD, Haase-Kromwijk BJ, et al. Hurdles, barriers, and successes of a national living donor kidney exchange program. Transplantation 2008; 86: 1749-1753.
- 5. Montgomery RA, Zachary AA, Ratner LE, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA 2005; 294: 1655-1663.
- 6. Hanto RL, Reitsma W, Delmonico FL. The development of a successful multiregional kidney paired donation program. Transplantation 2008; 86: 1744-1748.
- 7. Matas AJ, Garvey CA, Jacobs CL, Kahn JP. Nondirected donation of kidneys from living donors. N Engl J Med 2000; 343: 433-436.
- 8. Montgomery RA, Gentry SE, Marks WH, et al. Domino paired kidney donation: a strategy to make best use of live non-directed donation. Lancet 2006; 368: 419-421.
- 9. Tyden G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant 2005; 5: 145-148.
- 10. Delmonico FL. Exchanging kidneys — advances in living-donor transplantation. N Engl J Med 2004; 350: 1812-1814.
- 11. Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med 2009; 360: 459-469.





Abstract
Falling numbers of deceased organ donors and longer kidney transplant waiting lists have increased the emphasis on live kidney donation to meet demand for kidney transplantation.
Several new strategies have been introduced to expand live donation beyond the classic direct donation. These include:
altruistic donation;
paired kidney exchange (PKE); and
altruistic donor chains programs.
Using incompatible donor–recipient pairs and altruistic donors, the Western Australian PKE program achieved nine successful kidney transplantations between October 2007 and November 2008.
If PKE were performed routinely in Australia, the rate of kidney transplants could increase by 7%–10%.