Exercise-induced asthma (EIA) is the term used to describe the transient reduction in lung function that occurs after vigorous exercise.1 A reduction in FEV1 of 13.2% or more after exercise is diagnostic of EIA.2 A prevalence of EIA of between 19.3% and 23.4% has been reported in Australian schoolchildren.3,4 While severity of EIA was found to be associated with the number of wheeze attacks per year, 40% of children identified with EIA had not been diagnosed with asthma by a doctor.3
Exercise is the most common stimulus for inducing an attack in asthmatic children. Recognition of EIA is important, because full participation in sporting activities is a goal in the management of childhood asthma.
EIA is not predicted by risk factors, including positive answers to items about asthma in questionnaires,5 nor is it excluded by failure to report symptoms. In a child with undiagnosed EIA, the enquiry that has the most potential to elicit a positive response and thus identify EIA is "Do you feel more breathless/wheezy/symptomatic five to ten minutes after you stop exercise than during exercise."
There are many reasons why EIA is not recognised. Firstly, it occurs most commonly after exercise, so it may not limit performance unless the child has moderate to severe asthma. Secondly, children may fail to notice the symptoms of EIA until they take part in organised or competitive sport. Thirdly, 50% of children become refractory to the bronchoconstricting effects of exercise when exercise is repeated within an hour or warm-up occurs before exercise. This refractoriness means that the association of exercise and "asthma" symptoms does not occur on all occasions when exercise is performed.
Most children with well-controlled asthma do not need medication before exercise.6 Thus, taking 400 μg of budesonide for 4 weeks reduced the severity of EIA to such an extent that less than 10% of the standard dose of terbutaline was required for complete prevention of EIA.
In a recent study of 57 children with asthma and mild EIA (25% fall in forced expiratory volume in one second [FEV1] after exercise) and a normal FEV1 % predicted, low doses of budesonide for 12 weeks significantly reduced the severity of EIA (Box 1).7 A study in children with moderate EIA (34% fall in FEV1 after exercise) showed that treatment with 100 μg or 250 μg of fluticasone for three weeks was equally effective in reducing severity of EIA.8 In a study of 19 children with severe EIA (55% fall in FEV1 after exercise), 100 μg budesonide daily for four weeks stabilised symptoms and peak flow variability, but EIA still occurred at this low dose.9 In this double-blind crossover study, in which each child received the active medication in a random sequence for four weeks, the severity of the EIA diminished with increasing doses of budesonide. Thus, the mean percentage fall in FEV1 was 55.4% after placebo, 25.7% after 100 μg/day, 20.1% after 200 μg/day, and 9.9% after 400 μg/day.9
These studies clearly show that children with normal spirometry can still have EIA,7 and that lack of symptoms or variability of peak expiratory flow at rest does not reflect control of the airways in response to exercise.9 Furthermore, these studies support the long-held impression that EIA is one of the last problems to disappear after treatment with steroids, and that it is likely to indicate that the inflammation of asthma is active.
Eosinophils are one of the major inflammatory cells present in asthma, and studies show that there are more eosinophils in the sputum of children with asthma and EIA compared with children with asthma without EIA (Box 2).10 Furthermore, a recent study reported an acute increase in the proportion of eosinophils in sputum in asthma patients with EIA, but not in those without EIA.11
The inflammatory cells most likely to be important in EIA are mast cells and eosinophils. The mediators potentially released by these cells include histamine, leukotrienes and prostaglandins. EIA is most commonly associated with positive skin tests and raised IgE levels.
Histamine antagonists modify the severity of EIA in children, but the effect is small compared with the protective effects of a β2-agonist, sodium cromoglycate, and nedocromil sodium. Daily use of long-acting β2-agonists in the management of EIA in children has recently been questioned,12 and reversal of a provoked attack of asthma, such as EIA, is slower when short-acting β2-agonists are used daily.13 Therefore, we need to know about alternative treatments for EIA.
Leukotrienes are important in sustaining the airway response to exercise,14 and leukotriene antagonists have been shown to be effective in reducing EIA. For example, although immediately after exercise FEV1 fell somewhat, the time to return to pre-exercise FEV1 was significantly shorter in children taking the leukotriene antagonist montelukast compared with those taking placebo (Box 3).14 Leukotriene antagonists have the advantage of being administered as tablets and are effective in protecting against EIA in children when given once daily,14 or even as a single dose. The duration of their protective effect is maintained with daily use, and this gives them an advantage over the long-acting β2-agonists15 and other less expensive medications used to prevent EIA.
Leukotrienes are generated by eosinophils as well as mast cells, and are more potent in causing the human airways to narrow than histamine. Thus, a thousandth of the dose of leukotriene is required to induce the same degree of airway narrowing as histamine. This potency difference may be important in understanding why some children with significant EIA are not hyperresponsive to inhaled histamine.3 Leukotrienes are released from eosinophils in response to an increase in osmolarity and could feasibly cause transient migration of eosinophils to the airways. While treatment with inhaled steroids decreases eosinophil number, steroids do not prevent the contractile effects of leukotrienes. Thus, EIA may still occur if there are sufficient cells left containing this potent mediator.
The importance of EIA as a marker of airway inflammation and active asthma may be better appreciated if EIA is described as follows:
1: The role of steroids in exercise-induced asthma
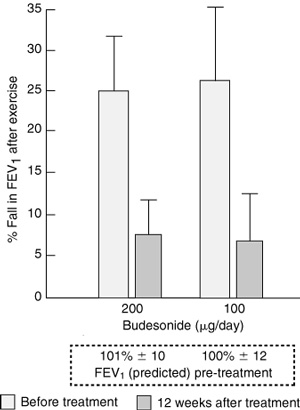
The mean (SD) fall in forced expiratory volume in one second (FEV1), expressed as a percentage of the pre-exercise value, after six minutes of running on a treadmill, before and 12 weeks after treatment with one dose daily of budesonide. There were 14 different children with exercise-induced asthma in each treatment group. The FEV1 measured before the start of treatment showed good lung function. (From Jonasson G, Carlsen KH, Hultquist C.7 Reproduced with permission from Blackwell Publishing.)
2: Increase in eosinophils in sputum of children with exercise-induced asthma (EIA)
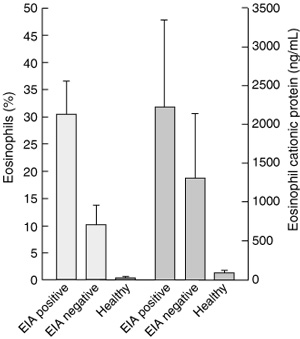
Proportion of eosinophils in induced sputum and eosinophil cationic protein content of sputum from 21 children with asthma with and without EIA (defined as > 20% fall in FEV1 after six minutes of running exercise), and nine healthy subjects. (Redrawn from Yoshikawa T, Shoji S, Fujii T, et al.10)
3: Effect of leukotriene antagonists in reducing exercise-induced asthma
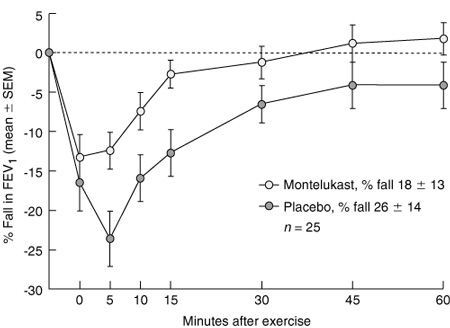
The mean (SEM) fall in forced expiratory volume in one second (FEV1) in 27 children, expressed as a percentage of the pre-exercise value after six minutes running on a treadmill. In this double-blind crossover trial, placebo or 5 mg montelukast was taken in the two evenings before the challenge. (From Kemp JP, Dockhorn RJ, Shapiro GG, et al.14 Reproduced with permission from Mosby Inc.)
- Sandra D Anderson1
- Department of Respiratory Medicine, Royal Prince Alfred Hospital, Camperdown, NSW.
- 1. Anderson SD. Exercise-induced asthma. In: Kay AB, editor. Allergy and allergic diseases. Oxford: Blackwell Scientific Publications; 1997: 692-711.
- 2. Godfrey S, Springer C, Bar-Yishay E, Avital A. Cut-off points defining normal and asthmatic bronchial reactivity to exercise and inhalation challenges in children and young adults. Eur Respir J 1999; 14: 659-668.
- 3. Haby MM, Peat JK, Mellis CM, et al. An exercise challenge for epidemiological studies of childhood asthma: validity and repeatability. Eur Respir J 1995; 8: 729-736.
- 4. Riedler J, Reade T, Dalton M, et al. Hypertonic saline challenge in an epidemiological survey of asthma in children. Am J Respir Crit Care Med 1994; 150: 1632-1639.
- 5. Rupp NT, Brudno DS, Guill MF. The value of screening for risk of exercise-induced asthma in high school athletes. Ann Allergy 1993; 70: 339-342.
- 6. Jonasson G, Carlsen KH, Jonasson C, Mowinckel P. Low-dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000; 55: 740-748.
- 7. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol 2000; 11: 120-125.
- 8. Hofstra WB, Neijens HJ, Duiverman EJ, et al. Dose-responses over time to inhaled fluticasone propionate: treatment of exercise- and methacholine-induced bronchoconstriction in children with asthma. Pediatr Pulmonol 2000; 29: 415-423.
- 9. Pedersen S, Hansen OR. Budesonide treatment of moderate and severe asthma in children: a dose-response study. J Allergy Clin Immunol 1995; 95(1 Pt 1): 29-33.
- 10. Yoshikawa T, Shoji S, Fujii T, et al. Severity of exercise-induced bronchoconstriction is related to airway eosinophilic inflammation in patients with asthma. Eur Respir J 1998; 12: 879-884.
- 11. Kivity S, Argaman A, Onn A, et al. Eosinophil influx into the airways in patients with exercise-induced asthma. Respir Med 2000; 94: 1200-1205.
- 12. Bisgaard H. Long-acting beta2-agonists in management of childhood asthma: a critical review of the literature. Pediatr Pulmonol 2000; 29: 221-234.
- 13. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am J Respir Crit Care Med 2002; 165: 1068-1070.
- 14. Kemp JP, Dockhorn RJ, Shapiro GG, et al. Montelukast once daily inhibits exercise-induced bronchoconstriction in 6- to 14-year-old children with asthma. J Pediatr 1998; 133: 424-428.
- 15. Villaran C, O'Neill SJ, Helbling A, et al. Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J Allergy Clin Immunol 1999; 104 (3 Pt 1): 547-553.





Abstract
What we knowWhat we need to know