The known: Estimated numbers of potentially preventable medication‐related hospitalisations in Australia at the population level have previously been reported only for veterans.
The new: We found high rates of potentially preventable medication‐related cardiovascular hospitalisations and emergency department presentations of Aboriginal and Torres Strait Islander people in Queensland.
The implications: Culturally appropriate and more targeted medication safety services are required to meet the population health needs of Aboriginal and Torres Strait Islander people.
Medicines are an important therapeutic tool for the treatment and management of chronic and preventable diseases in Aboriginal and Torres Strait Islander people. Medication‐related problems can be caused by a range of factors, including underuse, overuse, and inappropriate use.1,2,3 The most serious problems can result in hospitalisation or death. When there is a preventable component, these hospitalisations (defined as inpatient admissions and emergency department presentations) are termed potentially preventable medication‐related hospitalisations (PPMRHs).4,5,6 Although several Australian government policies for increasing access to subsidised medicines for Aboriginal and Torres Strait Islander people have been initiated in recent decades,7,8,9,10 other system‐based approaches to reducing medicine‐related harm are needed. For example, challenges to participating in Home Medicines Review related to cultural safety can be reduced by allowing people to choose the location of the service and inclusion of Aboriginal health workers.11,12,13,14,15 However, funding rules do not facilitate these options, despite promising results from two Australian government‐funded trials showing improved health outcomes.13,15 Inequitable access to Home Medicines Reviews and other medication safety services may contribute to poorer health outcomes for Aboriginal and Torres Strait Islander people.
Taking a needs‐based rather than an historical approach to health services planning (that is, assuming previous service delivery is sufficient) has gained momentum over the past two decades.16 This approach entails estimating the population need for services, then planning the health workforce response to meet this need. Estimating PPMRHs for Aboriginal and Torres Strait Islander populations is therefore an important step in assessing the need for medication safety services, identifying potential interventions, quantifying potential cost savings for the hospital system, and developing an evaluation framework. Such medication safety research has not previously been undertaken for Aboriginal and Torres Strait Islander peoples.
We therefore investigated the types and extent of medication‐related problems, as well as their ease of identification using administrative datasets. Our aim was to identify the numbers, proportions, and types of PPMRHs, deaths (within 30 days of PPMRHs), and hospital costs for Aboriginal and Torres Strait Islander people so that more targeted interventions — for example, quality improvement initiatives17 — can be implemented for the most frequent problems.
We report on a subset of clinical indicators that describe PPMRHs associated with cardiovascular disease (CVD), as these are a significant opportunity for intervention because of the high rates of potentially avoidable hospitalisations, health burden, morbidity, and mortality experienced by Aboriginal and Torres Strait Islander people.18 Our decision to focus on CVD was also pragmatic: these indicators can be robustly measured using the available data for a list of 81 previously identified indicators.5 In this way, we are using the CVD subset of clinical indicators to identify how a framework for investigating PPMRHs can be conceptualised.
Methods
We undertook an observational study because inequitable access to medication safety services for Aboriginal and Torres Strait Islander people has been identified;11,12 comparison with outcomes for non‐Indigenous people would risk a deficit discourse rather than describe the type and extent of medication problems. The study is reported in accordance with the STROBE checklist for cohort studies.19
Participants and setting
We extracted hospitalisations data from the Queensland Hospital Admitted Patient Data Collection, which contains information on all separations from public and licensed private hospitals, and from private day surgeries in Queensland. Emergency department data were from the Emergency Data Collection (EDC). Cost data for hospital episodes were provided by the Queensland Health National Hospital Cost Data Collection20 and were indexed relative to the base year of 2017 using the Reserve Bank of Australia Inflation Calculator (www.rba.gov.au/calculator). Data were extracted for all adults (18 years or older) who identified as Aboriginal or Torres Strait Islander people (www.health.qld.gov.au/hsu/atsihi) at at least one hospital separation during 1 January 2013 – 31 December 2017 (the latest data available at the time of application), understanding that the propensity to identify in the Queensland hospital system varies by presenting condition and a range of socio‐demographic variables.
Data sources
Hospital data were linked to Pharmaceutical Benefits Scheme (PBS) dispensing data, Medicare Benefits Schedule (MBS) service data, and National Death Index data by the Australian Institute of Health and Welfare using probabilistic matching. A de‐identified, linked dataset was provided to the researchers.
Outcome measures and variables
To estimate the number of PPMRHs, we used a list of clinical indicators appropriate for Aboriginal and Torres Strait Islander populations.5 This list was devised using a Delphi technique, including 13 panellists with experience in Aboriginal and Torres Strait Islander health, to revise a general population set of indicators based on clinical guidelines and the strength of the available evidence base.4,6 Of the 81 listed indicators, we used the nine CVD clinical indicators relevant to Aboriginal and Torres Strait Islander people5 (Box 1).
Eligible events included inpatient admissions and emergency department (ED) presentations, with some exceptions. People under 18 years of age at the time of admission or presentation were excluded. Transfers and statistical separations (episode changes) were excluded. Re‐admissions and repeat ED presentations on the same day with the same admission code were excluded. ED presentations were limited to those with outcomes marked as service event completed, discharged; died; or transferred to a short stay unit.
The dataset included primary and other causes for hospital admissions, but only the primary diagnosis was available for ED presentations. The nine clinical indicators (Box 1) covered suboptimal primary care whereby medication was indicated but not dispensed (eight indicators) and where medication was not indicated but dispensed (one indicator). Boolean logic was applied to identify instances in which the hospitalisation, specified by a set of International Statistical Classification of Diseases, tenth revision, Australian modification (ICD‐10‐AM) codes,23 was preceded by a pattern of suboptimal primary care consisting of the use or non‐use of certain medicines (proxied by PBS data on medicines dispensed). This meant that each hospitalisation event could meet the criteria for one or more PPMRH indicator and individual people might experience more than one PPMRH. Assumptions for the determination of PPMRH status are detailed in the Supporting Information, table 1. Events were first classified as meeting the criteria to be potentially avoidable (defined by a pre‐specified set of ICD‐10‐AM codes); for example, congestive heart failure or fluid overload as indicator 1. Additional criteria were then applied; for example, prior hospitalisation with or diagnosis of high blood pressure or congestive heart failure (indicator 1). The numerator was the number of events meeting the additional suboptimal medication status; for example, use of an agent known to exacerbate congestive heart failure at time of admission for indicator 1.
To identify whether a death may have been associated with a PPMRH, deaths within 30 days of the last known hospital discharge were evaluated. If the ICD‐10‐AM code for the cause of death was included in the list of codes for a PPMRH and the last known hospital event was coded with the same PPMRH, the death was classified as associated with the PPMRH, while acknowledging that causal inference is not possible.
Only limited information about individual participants was available from hospital records (age, sex recorded at birth, geographic location); prior diagnoses were inferred from earlier hospital admission coding. Information on other potential confounders, including system‐related factors such as health care quality, health care accessibility (apart from geographic location), and prescribing habits, was not available, nor on individual‐level factors that influence health care access, such as health beliefs, health literacy, and socio‐demographic characteristics (especially income and education). We therefore limited our analysis to being descriptive.
Statistical analysis
Descriptive statistics are presented according to variable type. The null hypothesis of no difference in the hospitalisation cost for PPMRHs and non‐PPMRHs was tested using the Wilcoxon rank sum test. For skewed data, medians and interquartile ranges (IQRs, reported as the difference between quartiles 1 and 3) are presented. Age group‐adjusted and sex‐adjusted PPMRH rates standardised to the Queensland Aboriginal and Torres Strait Islander population for each indicator across the included years were calculated using the direct method24 using Australian Bureau of Statistics population estimates and projections.25 All analyses were undertaken using Stata 16 in the Secure Unified Research Environment (SURE) maintained by the Sax Institute.
Places of usual residence (Statistical Area Level 2 codes) were converted to Accessibility/Remoteness Index of Australia (ARIA+) remoteness categories.26 As administrative data for prescriptions dispensed under Section 100 Remote Area Aboriginal Health Services Program provisions7 were not recorded, PPMRH numbers were expected to be overestimated for indicators for which omitted medicine is the key criterion (ie, the medicine is indicated but there is no dispensing record). This was expected to affect only remote and very remote area Queensland Health primary health care clinics and Aboriginal and Torres Strait Islander community‐controlled health services,7 and we included a spatial analysis to ascertain whether there was any evidence of such an effect. Total costs comprised direct and overhead costs for inpatient admissions and direct costs for ED separations.
Governance and positionality statement
We acknowledge the risks of non‐Indigenous‐led research, and deficit discourse in quantitative Indigenous health research, and reflect on the positioning of this article. This work was initiated through an earlier project, the Indigenous Medication Review Service (IMeRSe) study. This project was led by Aboriginal and Torres Strait Islander expert panel members (including the chair) and Aboriginal and Torres Strait Islander chief investigators and researchers (www.griffith.edu.au/menzies‐health‐institute‐queensland/our‐research/imerse‐project). Further funding and expansion of the IMeRSe service necessitates better understanding of the distribution of medication harms in Aboriginal and Torres Strait Islander populations and the need for medication review. Thus, this work emerged, and was initiated and funded as a joint venture with the Queensland Health Aboriginal and Torres Strait Islander Health Division. The authorship group for this article includes members of the IMeRSe team, including an Aboriginal and Torres Strait Islander senior author (DW), and non‐Indigenous authors working in Aboriginal and Torres Strait Islander primary care (WJ) and undertaking medication safety research that is purposefully inclusive of Aboriginal and Torres Strait Islander‐led health care (JS).
Ethics approval
The study was approved by the Griffith University Human Research Ethics Committee (2017/873), the AIHW Ethics Committee (EO2018/4/486), and the Queensland government (Public Health Act 2005 [Qld]; application reference number 180907). A waiver of consent was granted on the basis that the potential benefit to population health outcomes was justified, that informed consent was not practicable, and that the data were curated in the SURE environment.
Results
During 2013–2017, a total of 882 027 eligible hospital events involving 80 232 individuals in Queensland were identified, consisting of 288 720 ED presentations and 593 307 inpatient hospitalisations, of which 31 472 hospitalisations (8963 individuals) were CVD events as defined by at least one of the nine indicators (Box 2); 47 257 patients (59%) were women, the median age was 39 years (IQR, 27 years), and the median number of hospitalisations per person was two (IQR, 2) (Box 3).
The numbers of PPMRHs, length of stay, and deaths by cardiovascular indicator (meeting the admission criteria as well as suboptimal medication status) are reported in Box 4. For example, of the 7886 hospitalisations with congestive heart failure or fluid overload (indicator 1), 4350 (55.2%) were of people previously hospitalised with or who had diagnoses of high blood pressure or congestive heart failure (events meeting the admission criteria), of which 681 hospitalisations (15.7%) were associated with use of medicines known to exacerbate heart failure. For indicator 2, 1488 hospitalisations (34.2%) were associated with underuse of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or angiotensin receptor–neprilysin inhibitors. Of the 1089 hospitalisations of people with histories of acute coronary syndrome or previous myocardial infarction and admitted with myocardial infarction (indicator 3), 809 people (74.3%) were not receiving a recommended treatment (antiplatelet, beta blocker, or statin treatment). Of the 5417 hospitalisations of people with histories of diabetes and ischaemic events admitted with ischaemic events (indicator 9), 3343 (61.7%) were of people not receiving antiplatelet or lipid‐lowering therapy. The median length of hospital stay for PPMRHs by indicator ranged from 2 to 3 days. The numbers of PPMRH‐associated deaths within 30 days ranged from 5 to 66 by indicator. Of 4658 CVD deaths during included hospitalisations, 136 (2.9%) met the criteria for death following a PPMRH within 30 days.
The numbers of hospitalisations were lower in 2013 than in other years for all indicators except indicator 7, because of the effect of applying diagnosis history (eg, prior hospitalisation for congestive heart failure); first separations identified were omitted, resulting in lower counts for 2013. The number of hospitalisations generally increased over time; by indicator, 92–97% of hospitalisations were of people aged 40 years or older; 73–78% of PPMRHs were of people from moderately to highly accessible areas (Supporting Information, table 2).
No clear patterns in age group‐ and sex‐adjusted PPMRH rates standardised to the Queensland Aboriginal and Torres Strait Islander population for 2013–2017 over time were evident for any of the indicators (Supporting Information, table 3).
The median cost for PPMRHs during 2013–2017 was $4352 (IQR, $8742) and for non‐PPMRHs $4540 (IQR, $8359); the difference was not statistically significant. Median costs differed by indicator (Supporting Information, table 4), broadly in line with hospital length of stay (Box 4).
The PPMRH proportions were larger in areas of greater geographic accessibility for indicators 1 and 3, and in areas of greater remoteness for indicator 8 (Supporting Information, figure 1). The PPMRH proportions were highest overall in moderately accessible remoteness areas (range 25–39% by accessibility classification) for all indicators except indicators 6 and 7 (Supporting Information, table 2).
Discussion
Medication safety is the tenth National Health Priority Area in Australia.27 Nevertheless, information about the type and extent of medication problems in Australia is limited, including serious medication problems that result in hospitalisation or death. Our study was motivated by inequality of access to medication safety services in Australia, with more restricted access for Aboriginal and Torres Strait Islander people,11,12,13,14,15 and the need to reconsider how this problem might be overcome. By better understanding the pharmaco‐epidemiology of medication‐related problems and PPMRHs, effective, targeted, culturally appropriate services can be developed.16
Medication safety problems are multifactorial in nature and require system‐wide approaches to detecting, triaging, and managing the preventable component of risk. While this is true for all Australians, Aboriginal and Torres Strait Islander peoples face specific barriers that need to be considered. Our findings provide evidence of substantial morbidity and mortality associated with CVD medication‐related problems in Aboriginal and Torres Strait Islander people. While for some people medication according to clinical guidelines will not be appropriate (eg, because of an allergy), our findings suggest the need to improve health outcomes. Some people may not take recommended medicines for individual (eg, health beliefs, health literacy) or structural reasons (eg, access, cost, monitoring requirements).28
The relationship between geographic area and PPMRHs should be further investigated. For example, for indicator 1 (use of an agent known to exacerbate congestive heart failure at time of hospitalisation) the proportion of PPMRHs in rural and remote areas was smaller than elsewhere. This may indicate safer prescribing in these areas or more restricted access to primary health care. For indicator 3 (no use of an appropriate agent for CVD at time of hospitalisation), the proportion of PPMRHs was also smaller in rural and remote areas. Dispensing data for remote and very remote areas may not be complete because Section 100 supply is not recorded in the PBS dataset;7 however, this could also indicate safer prescribing or greater access to prescribed and dispensed medicines. These seemingly contradictory findings might be explained by other factors that result in lower PPMRH rates, such as quality improvement activities or the benefits of reduced access to multiple prescribers in less accessible areas. Most surprising were the larger PPMRH proportions in moderately accessible areas (except for indicators 6 and 7, no use of treatment to prevent acute coronary syndrome and transient ischaemic attack/ischaemic stroke). However, access to primary care in outer regional locations is poorer for Aboriginal and Torres Strait Islander people than for non‐Indigenous people, in part because of a lack of Indigenous‐specific services, as well as a general lack of general practitioner services.29
Our mixed findings indicate differences in access to primary care, but may also indicate that, where care is available, the use of multiple providers may be detrimental to health outcomes. Overall, our findings are consistent with those of the limited analyses previously undertaken in Australia;4,30,31 for example, in a retrospective analysis of data for Australian veterans (2004–2008), the PPMRH proportion meeting the admissions criteria was 20%, and the proportion of all separations 3%.4 We did not find consistent sex‐related differences in PPMRH proportions. Complex sex‐dependent interactions between CVD incidence and prevalence, health behaviour, health literacy, health beliefs, and other socio‐demographic factors are possible, but the data needed to examine such interactions were not available.
Limitations
Our data included all Aboriginal and Torres Strait Islander people in Queensland hospitalised during 2013–2017, and were obtained by linking administrative data that are highly representative of health care use. This is appropriate when taking the first steps towards needs‐based health services planning. However, as data on medicines dispensed under the Section 100 Remote Area Aboriginal Health Services Program7 were not available, we probably overestimated the number of PPMRHs in remote areas for indicators 2 to 9. As information on prior diagnoses was not available, we relied on information for the first recorded separation in the dataset to apply the admission criteria; this probably led to underestimation of the indicators and PPMRH proportions. These data limitations make it difficult to interpret temporal trends, such as the increase in PPMRHs over time. Analysis of data over a longer timeframe, closer to the present day and spanning the coronavirus disease 2019 (COVID‐19) pandemic, would allow more opportunity to detect changes in age‐ and sex‐adjusted. In addition, we had no access to clinical information about whether a recommended medicine had been considered but deemed inappropriate (because of allergy or intolerance, for example). Further, we did not have comparative information for people who experienced medication‐related problems who did not present to hospital, nor PPMRH rates for non‐Indigenous Australians. More detailed data that would facilitate causal analysis of PPMRHs were not available. Finally, tit is likely we underestimated the number of deaths, as datasets did not include people who died without presenting to an emergency department or being hospitalised.
Conclusion
We found that PPMRHs are a considerable health and health system burden for Aboriginal and Torres Strait Islander people. Recent research has indicated that many barriers can be overcome if medication safety services are tailored to Aboriginal and Torres Strait Islander people;13,14,15 indeed, much can be learned from the more holistic approaches to health care promoted in Aboriginal and Torres Strait Islander Health services. As new approaches to systematically improve medication safety in primary care emerge,17 health practitioners, researchers, and funding agencies must ensure that they are designed to include populations with the greatest health care needs, including Aboriginal and Torres Strait Islander peoples.
Box 1 – Clinical indicators of potentially preventable medication‐related hospitalisations (cardiovascular disease only)
|
Indicator |
Separation to avoid |
Admission criteria |
Suboptimal medication status |
||||||||||||
|
|
|||||||||||||||
|
1 |
Congestive heart failure/fluid overload |
Prior hospitalisation for or diagnosis of high blood pressure or congestive heart failure |
Use of an agent known to exacerbate congestive heart failure at time of hospitalisation* |
||||||||||||
|
2 |
Congestive heart failure/fluid overload |
Prior hospitalisation for or diagnosis of heart failure |
No use of angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker or angiotensin receptor–neprilysin inhibitor at time of hospitalisation |
||||||||||||
|
3 |
Myocardial infarction |
History of acute coronary syndrome or previous myocardial infarction |
No use of antiplatelet or beta blocker† or HMG‐CoA reductase inhibitor in the 3 months before hospitalisation |
||||||||||||
|
4 |
Myocardial infarction |
Insertion of stent within the previous 12 months |
No use of dual antiplatelet in the 2 months before admission |
||||||||||||
|
5 |
Thromboembolic cerebrovascular event |
Prior diagnosis of atrial fibrillation |
No use of anticoagulant in the 3 months before admission in a patient with high risk according to CHA2DS2‐VASc score |
||||||||||||
|
6 |
Acute coronary syndrome |
Cardiovascular disease risk known to be >15% before admission‡ |
No use of lipid‐lowering or antihypertensive therapy at time of hospitalisation |
||||||||||||
|
7 |
Transient ischaemic attack/ischaemic stroke |
Pulse quality or blood pressure not tested within previous 24 months§ |
No use of any of antiplatelet, antihypertensive, anticoagulant or lipid‐lowering therapy |
||||||||||||
|
8 |
Ischaemic coronary event |
History of angina or acute coronary syndrome |
No use of beta blocker, calcium channel blocker or nitrate |
||||||||||||
|
9 |
Ischaemic event |
History of diabetes and history of ischaemic event |
No use of antiplatelet or lipid‐lowering therapy at time of hospitalisation |
||||||||||||
|
|
|||||||||||||||
|
CHA2DS2‐VASc = a set of clinical prediction rules for estimating the risk of stroke in people with non‐rheumatic atrial fibrillation;21 HMG‐CoA = 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase; MBS = Medicare Benefits Schedule. * Including non‐steroidal anti‐inflammatory drugs, cyclooxygenase‐2 inhibitors, anti‐arrhythmics (apart from beta blockers or amiodarone), non‐dihydropyridine calcium channel blockers in systolic congestive heart failure (verapamil, diltiazem), corticosteroids, clozapine, tricyclic antidepressants, tyrosine kinase inhibitors, thiazolidinediones, and tumour necrosis factor antagonists. † In reduced left ventricular systolic function only. ‡ As adults over the age of 60 years with diabetes are equivalent to high risk (greater than 15%),22 we conservatively applied the criteria of being older than 60 years at the time of event and having a prior hospitalisation for diabetes complications to identify history of diabetes. § As a proxy measure for pulse quality or blood pressure not tested, we used the absence of MBS service item claims for any of the following items: health assessment for Aboriginal and Torres Strait Islander people (MBS item 715), health assessments (MBS items 701, 703. 707), and general practitioner level A–D consultations (MBS items 3, 4, 23, 36, 44). |
|||||||||||||||
Box 2 – Selection of potentially preventable medication‐related hospitalisations of Aboriginal or Torres Strait Islander people, Australia, 1 January 2013 – 31 December 2017
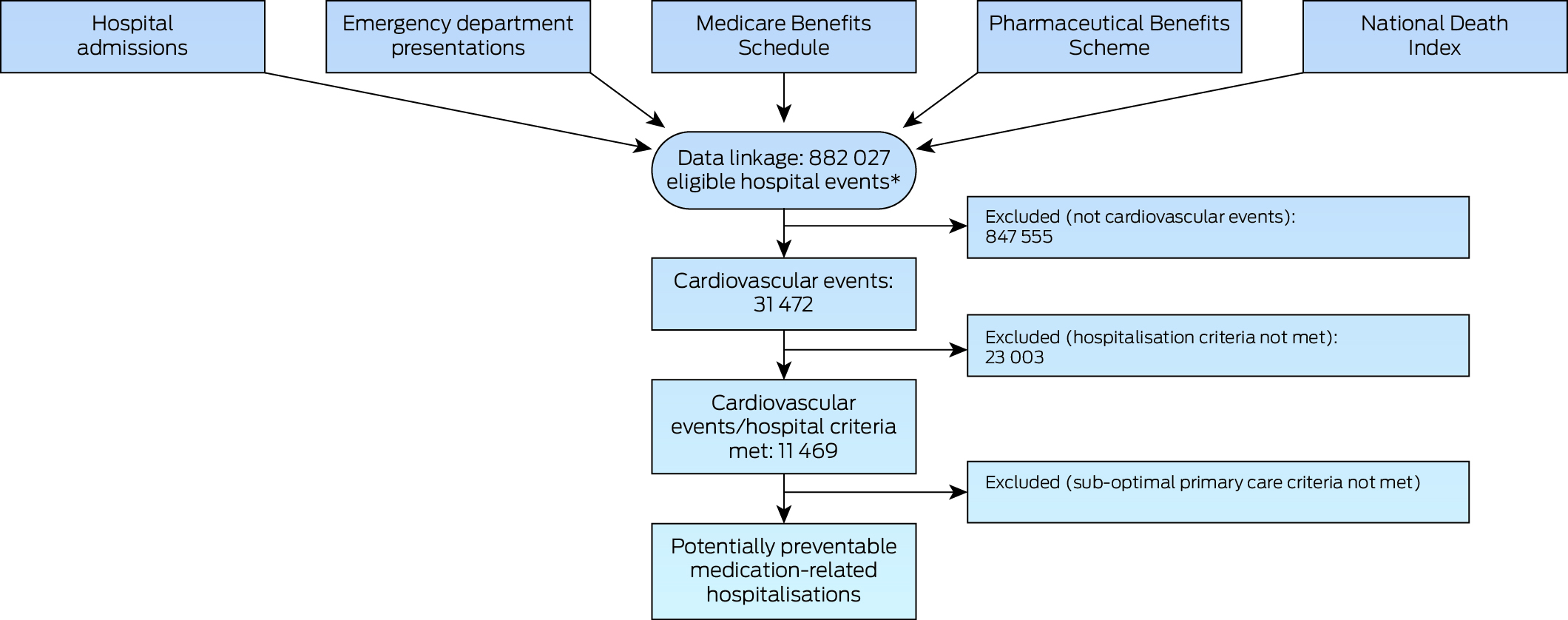
AIHW = Australian Institute of Health and Welfare. MBS = Medicare Benefits Schedule. PBS = Pharmaceutical Benefits Scheme. QHAPDC = Queensland Hospital Admitted Patient Data Collection.* Includes the service events completed, discharged; died; and transferred to a short stay unit (from Emergency Data Collection). Excludes 51 events for which linkage with MBS and PBS data were not possible.
Box 3 – Numbers of hospital admissions and emergency presentations (hospitalisation events), by year
|
Year |
Number of events |
Number of people |
Age (years), median (IQR) |
Events per person, median (IQR) |
|||||||||||
|
|
|||||||||||||||
|
Total |
882 027 |
80 232* |
39 (27) |
2 (2) |
|||||||||||
|
2013 |
155 897 |
36 807 |
37 (27) |
2 (2) |
|||||||||||
|
2014 |
173 308 |
39 844 |
38 (26) |
2 (3) |
|||||||||||
|
2015 |
177 768 |
41 191 |
38 (27) |
2 (2) |
|||||||||||
|
2016 |
181 593 |
42 248 |
39 (27) |
2 (2) |
|||||||||||
|
2017 |
193 461 |
43 424 |
40 (27) |
2 (2) |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Total number of individuals (multiple admissions possible). |
|||||||||||||||
Box 4 – Numbers of potentially preventable medication‐related hospitalisations, length of stay and deaths, by cardiovascular indicator, 2013–2017
|
Indicator |
Separation to avoid |
Number of separations |
Admission criteria |
Separations that met criteria |
PPMRHs, number with suboptimal medication status (proportion that met criteria) |
Length of stay (days), median (IQR) |
Deaths |
||||||||
|
|
|||||||||||||||
|
Total* |
— |
31 472 |
— |
11 469 |
— |
— |
136 |
||||||||
|
1 |
Congestive heart failure/fluid overload |
7886 |
Prior hospitalisation for or diagnosis of high blood pressure or congestive heart failure |
4350 |
681 (15.7%) |
3 (6.0) |
14 |
||||||||
|
2 |
Congestive heart failure/fluid overload |
7886 |
Prior hospitalisation for or diagnosis of congestive heart failure |
4350 |
1488 (34.2%) |
3 (6.0) |
14 |
||||||||
|
3 |
Myocardial infarction |
3838 |
History of acute coronary syndrome or previous myocardial infarction |
1089 |
809 (74.3%) |
3 (5.0) |
20 |
||||||||
|
4 |
Myocardial infarction |
3838 |
Insertion of stent within the previous 12 months |
371 |
170 (45.8%) |
2 (4.0) |
5 |
||||||||
|
5 |
Thromboembolic cerebrovascular event |
2282 |
Prior diagnosis of atrial fibrillation |
400 |
81 (20.3%) |
3 (6.5) |
13 |
||||||||
|
6 |
Acute coronary syndrome |
7907 |
Cardiovascular disease risk known to be >15% before hospitalisation |
815 |
180 (22.1%) |
3 (5.0) |
23 |
||||||||
|
7 |
Transient ischaemic attack/ischaemic stroke |
2282 |
Pulse quality or blood pressure not tested within previous 24 months |
2192 |
1236 (56.4%) |
2 (5.0) |
45 |
||||||||
|
8 |
Ischaemic coronary event |
9776 |
History of angina or acute coronary syndrome |
2586 |
986 (38.1%) |
2 (3.0) |
18 |
||||||||
|
9 |
Ischaemic event |
12 058 |
History of diabetes and history of ischaemic event |
5417 |
3343 (61.7%) |
2 (4.0) |
66 |
||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; PPMRH = potentially preventable medication‐related hospitalisation. * An event could be coded as meeting the criteria for multiple indicators. |
|||||||||||||||
Received 13 March 2023, accepted 29 July 2024
- Jean Spinks1
- Gabor Mihala1
- Warren Jennings1,2
- Robert S Ware3
- Lisa M Kalisch Ellett4
- Elizabeth E Roughead4
- Daniel Williamson5
- 1 University of Queensland, Brisbane, QLD
- 2 Metro South Hospital and Health Service, Brisbane, QLD
- 3 Griffith University, Gold Coast, QLD
- 4 University of South Australia, Adelaide, SA
- 5 Queensland Health, Brisbane, QLD
Open access:
Open access publishing facilitated by the University of Queensland, as part of the Wiley – the University of Queensland agreement via the Council of Australian University Librarians.
Data Sharing:
Secondary (administrative) linked data were used for this analysis made available by Queensland Health (hospital admissions, emergency department presentations, costs), and the Australian Department of Health and Aged Care (Medical Benefits Scheme, Pharmaceutical Benefits Scheme and the National Death Index). Analysis of this data was at all times conducted in accordance with the ethical approval provided by the data custodians.
This research was supported by the Queensland Health Aboriginal and Torres Strait Islander Health Division and Menzies Health Institute Queensland. We undertook the analysis independent of the funding organisations.
Daniel Williamson works for Queensland Health. Apart from his contribution, the funding organisations did not have a role in the study design, analysis and reporting or the publication of this study.
- 1. Pharmaceutical Care Network Europe Association. Classification for drug related problems v9.00. 2019. https://www.pcne.org/upload/files/334_PCNE_classification_V9‐0.pdf (viewed Oct 2023).
- 2. Williams M, Peterson G, Tenni P, et al. DOCUMENT: a system for classifying drug‐related problems in community pharmacy. Int J Clin Pharm 2012; 34: 43‐52.
- 3. Nicosia FM, Spar M, Stebbins M, et al. What is a medication‐related problem? A qualitative study of older adults and primary care clinicians. J Gen Intern Med 2020; 35: 724‐731.
- 4. Kalisch LM, Caughey G, Barratt J, et al. Prevalence of preventable medication‐related hospitalizations in Australia: an opportunity to reduce harm. Int J Qual Health Care 2012; 24: 239‐249.
- 5. Spinks J, Kalisch Ellett L, Spurling G, et al. Adaptation of potentially preventable medication‐related hospitalisation indicators for Indigenous populations in Australia using a modified Delphi technique. BMJ Open 2019; 9:e031369.
- 6. Caughey GE, Kalisch Ellett LM, Wong TY. Development of evidence‐based Australian medication‐related indicators of potentially preventable hospitalisations: a modified RAND appropriateness method. BMJ Open 2014; 4: e004625.
- 7. Australian Department of Health and Aged Care. Reporting for the Remote Area Aboriginal Health Services Program. Updated 27 Sept 2024. https://www.health.gov.au/our‐work/raahs‐program/reporting?utm_source=health.gov.au&utm_medium=callout‐auto‐custom&utm_campaign=digital_transformation (viewed Oct 2024).
- 8. Pharmacy Programs Administrator. Program rules: Quality Use of Medicines Maximised for Aboriginal and Torres Strait Islander People (QUMAX). July 2020. https://www.ppaonline.com.au/wp‐content/uploads/2019/01/QUMAX‐Program‐Rules.pdf (viewed Oct 2023).
- 9. Services Australia. Closing the Gap PBS co‐payment for health professionals. 1 July 2024. https://www.servicesaustralia.gov.au/closing‐gap‐pbs‐co‐payment‐for‐health‐professionals (viewed Oct 2024).
- 10. Pharmacy Programs Administrator. Indigenous Health Services Pharmacy Support Program. Undated. https://www.ppaonline.com.au/programs/aboriginal‐and‐torres‐strait‐islander/indigenous‐health‐services‐pharmacy‐support‐program (viewed Oct 2023).
- 11. Swain LS, Barclay L. Exploration of Aboriginal and Torres Strait Islander perspectives of Home Medicines Review. Rural Remote Health 2015; 15: 3009.
- 12. Swain L, Barclay L. Medication reviews are useful, but the model needs to be changed: perspectives of Aboriginal Health Service health professionals on Home Medicines Reviews. BMC Health Serv Res 2015; 15: 366.
- 13. Wheeler AJ, Spinks J, Kelly F, et al. Improved medication management for Aboriginal and Torres Strait Islanders through pharmacist advice and culturally appropriate services: a feasibility study of an Indigenous Medication Review Service (IMeRSe) in Australia. Final report. Dec 2020 [unpublished report].
- 14. Wheeler AJ, Spinks J, Kelly F, et al. Protocol for a feasibility study of an Indigenous Medication Review Service (IMeRSe) in Australia. BMJ Open 2018; 8: e026462.
- 15. Couzos S, Smith D, Stephens M, et al. Integrating pharmacists into Aboriginal community controlled health services (IPAC project): protocol for an interventional, non‐randomised study to improve chronic disease outcomes. Res Social Adm Pharm 2020; 16: 1431‐1441.
- 16. Birch S, Gibson J, McBride A, et al. Opportunities for, and implications of, skill mix changes in health care pathways: pay, productivity and practice variations in a needs‐based planning framework. Soc Sci Med 2020; 250: 112863.
- 17. Spinks J, Violette R, Boyle D, et al. Activating pharmacists to reduce the frequency of medication‐related problems (ACTMed): a stepped wedge cluster randomised trial. Med J Aust 2023; 219: 325‐331. https://www.mja.com.au/journal/2023/219/7/activating‐pharmacists‐reduce‐frequency‐medication‐related‐problems‐actmed
- 18. Agostino J, Wong D, Paige E, et al. Cardiovascular disease risk assessment for Aboriginal and Torres Strait Islander adults aged under 35 years: a consensus statement. Med J Aust 2020; 212: 422‐427. https://www.mja.com.au/journal/2020/212/9/cardiovascular‐disease‐risk‐assessment‐aboriginal‐and‐torres‐strait‐islander
- 19. Strengthening the reporting of observational studies in epidemiology. STROBE checklist: cohort studies. https://www.strobe‐statement.org/checklists (viewed Oct 2023).
- 20. Queensland Health National Hospital Cost Data Collection. Commonly requested data items [form]. https://www.health.qld.gov.au/__data/assets/pdf_file/0032/834188/crdi‐nhcdc.pdf (viewed Oct 2023).
- 21. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137: 263‐272.
- 22. Heart Foundation. Australian cardiovascular risk charts. 2018. Archived: https://web.archive.org/web/20200316052119/https://www.heartfoundation.org.au/images/uploads/publications/Absolute‐CVD‐Risk‐Quick‐Reference‐Guide_2018.pdf (viewed Mar 2024).
- 23. Independent Health and Aged Care Pricing Authority. ICD‐10‐AM/ACHI/ACS tenth edition. 2017. https://www.ihacpa.gov.au/resources/icd‐10‐amachiacs‐tenth‐edition (viewed Oct 2023).
- 24. Australian Institute of Health and Welfare. Principles on the use of direct age‐standardisation in administrative data collections: for measuring the gap between Indigenous and non‐Indigenous Australians (AIHW cat. no. CSI 12). 7 Oct 2011. https://www.aihw.gov.au/reports/indigenous‐australians/principles‐on‐the‐use‐of‐direct‐age‐standardisatio/summary (viewed Mar 2024).
- 25. Australian Bureau of Statistics. Estimated population, Aboriginal and Torres Strait Islander Australians, Australia, states and territories, 2001–2011. In: Estimates and projections, Aboriginal and Torres Strait Islander Australians, 2001 to 2026 (3238.0). 30 Apr 2014. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3238.02001%20to%202026?OpenDocument (viewed Mar 2024).
- 26. Australian Centre for Housing Research. Accessibility/Remoteness Index of Australia (ARIA+). Updated 22 Mar 2023. https://able.adelaide.edu.au/housing‐research/data‐gateway/aria (viewed Oct 2023).
- 27. Australian Commission on Safety and Quality in Health Care. Quality use of medicines. 2020. https://www.safetyandquality.gov.au/publications‐and‐resources/resource‐library/quality‐use‐medicines‐and‐medicines‐safety‐discussion‐paper (viewed June 2024).
- 28. Cross AJ, Elliott RA, Petrie K, et al. Interventions for improving medication‐taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev 2020; CD012419.
- 29. Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander Health Performance Framework summary report 2020 (cat. no. IHPF 2). https://www.indigenoushpf.gov.au/publications/hpf‐summary‐2020 (viewed Oct 2023).
- 30. Roughead L, Semple S, Rosenfeld E. Literature review: medication safety in Australia. Sydney: Australian Commission on Safety and Quality in Health Care, 2013. https://www.safetyandquality.gov.au/publications‐and‐resources/resource‐library/literature‐review‐medication‐safety‐australia (viewed June 2024).
- 31. Lim R, Kalisch Ellett L, Semple S, et al. The extent of medication‐related hospital admissions in Australia: a review from 1988 to 2021. Drug Saf 2022: 45: 249‐257.






Abstract
Objective: To identify the proportion of hospitalisations (inpatient admissions and emergency department presentations) of Aboriginal and Torres Strait Islander people in Queensland that were medication‐related and potentially preventable for nine clinical indicators of cardiovascular disease (CVD).
Study design: Retrospective cohort study; analysis of linked hospitalisations and emergency department presentations data and administrative records of medical services, pharmaceuticals, and deaths.
Setting, participants: Aboriginal or Torres Strait Islander adults (18 years or older) admitted to Queensland public and private hospitals, 1 January 2013 – 31 December 2017.
Main outcome measures: Potentially preventable medication‐related hospitalisations (PPMRHs), defined by a set of clinical indicators describing CVD; deaths within 30 days of PPMRHs; hospital costs.
Results: We identified 31 472 CVD‐related hospitalisations, of which 11 469 were of people with medical histories suggesting harm that was foreseeable and preventable with appropriate treatment. Of the 7886 hospitalisations with congestive heart failure, 4350 (55%) were of people with prior CVD diagnoses; 681 (16%) were associated with use of medicines known to exacerbate congestive heart failure, and 1488 (34%) were associated with underuse of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or angiotensin receptor–neprilysin inhibitors. Of the 1089 hospitalisations with myocardial infarction of people who had previously experienced myocardial infarction or acute coronary syndrome events, 809 (74%) were not receiving recommended treatment at the time of hospitalisation. Of the 5417 hospitalisations with ischaemic events of people with histories including diabetes and earlier ischaemic events, 3343 (62%) were not receiving antiplatelet or lipid‐lowering therapy. The median cost associated with PPMRHs for the time period (2013–2017) was $4352 (interquartile range, $8742), and 136 (3%) of CVD‐related deaths within 30 days of hospital discharge followed PPMRH events.
Conclusions: Interventions supporting targeted and timely medication safety services for Aboriginal and Torres Strait Islander people need to be reviewed and improved to reduce the numbers of avoidable hospitalisations and deaths.