The known: The 2020 Australian guideline‐recommended treatment intervals for women diagnosed with breast cancer are based largely upon expert consensus.
The new: The risk of death from breast cancer was 43% higher for women for whom at least one treatment interval was longer than recommended than for women who received treatment within the guideline timeframes. The most critical intervals were diagnosis to surgery, surgery to chemotherapy, and chemotherapy to radiotherapy.
The implications: Our findings highlight the importance of timely treatment for women with breast cancer, and support the treatment timeframes recommended in the 2020 guideline.
Breast cancer is the second most frequent cause (after lung cancer) of cancer‐related deaths of Australian women.1 Despite efforts to improve therapy, 33–52% of women diagnosed with breast cancer do not receive timely treatment,2,3 and there is increasing evidence that delay in commencing treatment is associated with poorer survival.4,5
Cancer Australia updated its treatment guidelines for early breast cancer in 2020.6 The guidelines now include recommendations regarding six treatment intervals for women with early breast cancer (stages I–III): diagnosis to neoadjuvant therapy, neoadjuvant therapy to surgery, diagnosis to surgery, surgery to chemotherapy, surgery to radiotherapy, and chemotherapy to radiotherapy7 (Box 1).
A recent Queensland study found that survival was better for women who completed the treatment pathway within 37 weeks of breast cancer diagnosis than for those who did not.5 The analysis was restricted to the fewer than 50% of women with breast cancer whose treatment comprised surgery, chemotherapy, and radiotherapy (in this order). In the study we report in this article, we investigated the association between guideline‐recommended treatment intervals and breast cancer survival, separately for each phase of treatment, in a large population‐based cohort of women diagnosed with early breast cancer.
Methods
The Breast Cancer Outcomes Study is a population‐based cohort study of women aged 20–79 years diagnosed with invasive breast cancer during 1 March 2010 – 30 June 2013, as recorded in the Queensland Cancer Register. The complete study protocol has been published elsewhere.10 Deaths information to 31 December 2020 was obtained by the Queensland Cancer Register using internal linkage with the Queensland Register of Birth, Deaths, and Marriages, and external linkage with the National Death Index by the Australian Institute of Health and Welfare. Further information was collected from participants in validated, semi‐structured telephone interviews by trained health interviewers. Telephone interviews were undertaken within three years of diagnosis (median, 391 days; interquartile range, 276–890 days; range, 93–923 days). Demographic and clinical information were obtained from telephone interviews and by linking Queensland Cancer Registry data with patient medical records (manually extracted) (Supporting Information, box 2).
Treatment intervals
We compared the following treatment intervals for each participating woman, as applicable, with the recommendations in the 2020 Australian treatment guidelines6 (Box 1):
- diagnosis to commencement of neoadjuvant systemic therapy;
- completion of neoadjuvant systemic therapy to surgery;
- diagnosis to surgery (for women who did not receive neoadjuvant systemic therapy);
- surgery to commencement of adjuvant chemotherapy;
- surgery to commencement of adjuvant radiotherapy (for women who did not receive adjuvant chemotherapy); and
- completion of adjuvant chemotherapy to commencement of adjuvant radiotherapy.
“Guideline non‐compliance” was defined as the treatment interval exceeding the recommended length, “guideline compliance” as falling within the recommended interval. “Overall guideline non‐compliance” was defined as non‐compliance with at least one guideline recommendation, “overall guideline compliance” as guideline recommendations for all relevant treatment intervals being met. As the date of the decision to treat was unavailable for interval 3 (diagnosis to surgery), we assumed that decision making required 15 days5 and modified the criterion to “surgery within 45 days of diagnosis”. The number of treatments a woman received was also recorded, as this affects the number of treatment intervals.
Survival analysis
The primary outcome was breast cancer‐specific survival. Death from breast cancer was defined as the cause of death being recorded with the International Classification of Diseases, tenth revision (ICD‐10) code C50.
We assessed survival differences between women in the “guideline compliance” and “guideline non‐compliance” groups, by interval, in flexible parametric survival models.11 Age at diagnosis, tumour stage, grade, and mode of detection — recognised prognostic factors for breast cancer12 — were included as parameters; in the “overall guideline compliance” model, number of treatments (one, more than one) was also included. The models of best fit, based on the optimal number of knots, were determined using the Akaike information criterion, the Bayes information criterion, and the likelihood ratio statistic.13 We report adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) and survival difference curves. For the survival analysis we employed the stpm2 function for model building and standsurv function for survival difference and hazard ratio estimation in Stata/SE 17.0.11
A sensitivity survival analysis was further adjusted for socio‐demographic factors previously found to not influence breast cancer survival in our cohort (family history, smoking, marital status, annual income, private insurance status, residential remoteness).12
Optimal cut‐points
The optimal cut‐points (that is, the time point in a treatment interval with the greatest discrimination ability for defining statistically poorer survival) for intervals 3 to 6 were estimated from the survival and hazard ratio curves, using the minimum P value method (cut‐point with lowest P value and Bonferroni adjusted P < 0.20 selected as optimal).14 Optimal cut‐points were not estimated for intervals 1 and 2 because the number of eligible women was small (further details: Supporting Information, box 3).
Characteristics of women in the guideline non‐compliance group
We assessed associations of the characteristics of women for whom guideline non‐compliance was identified in multivariable logistic regression models. Data for all factors (Supporting Information, box 2) were initially included and then excluded by backward stepwise selection;15 variables for which P > 0.20 in likelihood ratio tests were omitted. At each step, variables previously removed from the model were tested for their eligibility to be re‐included. We report adjusted odds ratios (aORs) with 95% CIs.
The level of missing data for socio‐demographic variables ranged from 0 to 11% (Supporting Information, table 1). As a complete case analysis would have excluded 17% of the cohort, potentially introducing bias, missing data were imputed using multiple imputation16 with the Stata mi impute chained and mi estimate commands for chained equations and subsequent regression model estimation. We included all variables in the final model, and auxiliary variables correlated with missing variables (Pearson r > 0.4). Based on the proportion of incomplete cases,17 we performed eighteen imputations for the logistic models.
Ethics approval
The human research ethics committee of Griffith University approved the study (PSY/C4/09/HREC).
Results
A total of 5426 women were eligible for our study, of whom 66 had died by the time of ascertainment. The general practitioners of 4672 women (87%) provided consent to contact the women, of whom 3326 (71%) subsequently completed interviews. Information was incomplete for 250 interviewees, and their data were excluded from our analysis, as were data for thirty women with stage IV disease and two women who did not undergo surgery (Supporting Information, figure 1). The age distributions of the 3044 included women (56.1% of all eligible women; 65.1% of women invited to participate in interviews) and the women whose data were excluded were similar, but women living in major cities were less likely to participate in the study (data not shown).
One or more instances of non‐compliance with guideline interval recommendations were identified for 1375 women (45%); the proportions differed by the characteristics of the women (Box 2). The proportion of women for whom guideline non‐compliance was identified was largest for surgery to radiotherapy (interval 5: 525 of 1071, 49%) and smallest for diagnosis to surgery (interval 3: 135 of 2972, 4.5%) (Box 3).
Guideline compliance and survival
The median follow‐up time from diagnosis (to 31 December 2020) was 105 months (interquartile range, 11–129 months). During the follow‐up period, 165 women (5.4%) died from breast cancer.
The risk of death from breast cancer during the study follow‐up was greater for women in the overall guideline non‐compliance group than for those in the overall guideline compliance group (aHR, 1.43; 95% CI, 1.04–1.96) (Supporting Information, table 3). The survival difference was not statistically significant during the five years following diagnosis; ten years after diagnosis, the difference was 1.9 percentage points lower for women in the non‐compliance group (92.5% v 94.4%) (Box 4).
In sensitivity survival analyses including additional socio‐demographic factors in the survival model, the results for both the overall guideline compliance and non‐compliance groups were similar to those in the main model (Supporting Information, figure 2).
The adjusted risk of death from breast cancer during study follow‐up was greater for women for whom interval 3 (diagnosis to surgery) exceeded 45 days (aHR, 2.14; 95% CI, 1.18–3.89), and survival ten years after diagnosis was 4.4 percentage points lower (89.8% v 94.2%). The adjusted risk of death from breast cancer was greater for women for whom interval 6 (chemotherapy to radiotherapy) exceeded 28 days (aHR, 1.62; 95% CI, 1.06–2.47), and survival ten years after diagnosis was 3.8 percentage points lower (88.7% v 92.5%). Survival outcomes were not significantly influenced by compliance with recommendations for the other four intervals (Supporting Information, table 3; Box 5).
Optimal treatment interval cut‐points
The risk of death from breast cancer was significantly greater for women who underwent surgery more than 29 days after diagnosis (aHR, 1.76; 95% CI, 1.19–2.59), commenced adjuvant chemotherapy more than 36 days after surgery (aHR, 1.63; 95% CI, 1.13–2.36), or commenced radiotherapy more than 31 days after completing adjuvant chemotherapy (aHR, 1.83; 95% CI, 1.19–2.80) than for those who received treatment before the corresponding time points; no optimal cut‐ point could be determined for interval 5 (surgery to radiotherapy) (Box 6).
Characteristics of women with extended treatment intervals
Treatment intervals longer than recommended were more frequent among women for whom breast cancer was detected by screening in public facilities (aOR, 1.58; 95% CI, 1.22–2.04) or according to symptoms (aOR, 1.39; 95% CI, 1.09–1.79) than for women with breast cancer detected by screening in private facilities. Treatment intervals longer than recommended were also more frequent for women who completed or started treatment in December or January rather than February–November (aOR, 2.05; 95% CI, 1.75–2.40), and for women who did not have family histories of breast or ovarian cancer (aOR, 1.22; 95% CI, 1.04–1.43), were current smokers (aOR, 1.41; 95% CI, 1.05–1.90), with annual incomes below $52 000 (aOR, 1.30; 95% CI, 1.01–1.67), did not have private health insurance (aOR, 1.96; 95% CI, 1.66–2.32), or did not live in major cities (aOR, 1.38; 95% CI, 1.18–1.62) (Box 7).
Discussion
In 2020, Cancer Australia updated their guidelines for early breast cancer treatment and recommended timeframes for six treatment intervals. We analysed population‐based data to evaluate these recommendations with respect to breast cancer survival. Our analysis, based on dividing the treatment pathway into its component intervals, provided results relevant to all women with early breast cancer receiving various treatment combinations. We report evidence that survival was significantly better for women who received timely treatment than for those who did not.
Interval 3: Diagnosis to surgery
The Australian treatment guideline recommends “surgery within 1 month of a decision to treat with surgery among those patients who do not receive neoadjuvant therapy”6 (interval 3). This recommendation was based on expert consensus because no evidence‐based recommendation was identified for this topic, but longer diagnosis‐to‐surgery times have been associated with reduced overall survival.21,22 No studies reporting evidence relevant to how soon a woman should undergo surgery after diagnosis have been published. We found that survival was statistically better for women treated within 45 days of diagnosis (our modification of the guidelines), but a cut‐point of 29 days provided better discrimination; that is, survival for women who underwent surgery more than 29 days after diagnosis was significantly poorer. Health system practices and hospital burden, as well as factors such as geographic and cultural diversity, probably influence the timeliness of breast cancer care. Opportunities for shortening the diagnosis‐to‐treatment window while maintaining quality of care may be facilitated by digital health care innovations integrated with person‐centred care and a survivorship approach.23
Interval 4: Surgery to chemotherapy
The Australian guideline recommendation for the time between surgery and chemotherapy (interval 4) was based on European Society for Medical Oncology (ESMO)8 and Royal Australasian College of Physicians (RACP) guidelines.9 The ESMO guidelines recommend that “adjuvant systemic treatment should preferably start within 3–6 weeks after surgery”, based on level I, grade A study evidence (Infectious Diseases Society of America–United States Public Health Service grading system); the RACP guidelines advise that “adjuvant chemotherapy should commence within 4 weeks of the date of surgery”, based on level III, grade C study evidence (National Health and Medical Research Council grading system). Neither guideline specifies the studies upon which their recommendations were based. The findings of our population‐based study suggest that survival was significantly better for women who commenced adjuvant chemotherapy within 36 days of surgery (ie, within the recommended timeframe).
Interval 5: Surgery to radiotherapy
Although the Australian guideline recommends commencing radiation therapy within eight weeks of surgery for women who do not receive adjuvant chemotherapy, we did not identify a significant influence on breast cancer‐specific survival of the surgery‐to‐radiotherapy interval (interval 5) (to 103 days, or about fifteen weeks). This finding is consistent with studies that did not find survival differences to twelve,24 sixteen,25 or twenty weeks26 for this interval, but poorer survival has been reported for longer intervals.24,26 However, one recent study found that survival was better for women who commenced radiotherapy within eight weeks of lumpectomy.27 Our finding regarding the surgery‐to‐radiotherapy interval may have been influenced by unrecognised confounders, but we included a broad variety of factors in our model. Uncertainty consequently remains about the optimal treatment interval between surgery and radiotherapy.
Interval 6: Chemotherapy to radiotherapy
Several studies have evaluated the timing of radiotherapy for women who underwent only surgery and radiotherapy,24,25,26 but their findings may not be applicable to patients who also receive chemotherapy. We differentiated between women who did not receive chemotherapy, assessing the surgery‐to‐radiotherapy interval (interval 5), and those who did, assessing the chemotherapy‐to‐radiotherapy interval (interval 6). Based on expert advice, the Australian guideline recommends that women commence radiation therapy within four weeks of completing adjuvant chemotherapy (interval 6). Two recent studies in China found negative associations between longer chemotherapy‐to‐radiotherapy interval and survival, estimating cut‐points of twelve weeks28 and six weeks.29 We found that survival was significantly better for women who underwent radiotherapy within four weeks of chemotherapy (ie, as recommended by the Australian guideline), but the optimal cut‐point analysis indicated that discrimination was better with the slightly later cut‐point of 31 days.
An unadjusted log‐rank analysis of Queensland Health data for 2010–2019 suggested an association between time to treatment completion and breast cancer survival, finding that survival was better for women who completed surgery, chemotherapy, and radiotherapy (in this order) within 37 weeks of diagnosis than with longer treatment times.5 Our similar analysis found a similar pattern, but the association was not statistically significant after adjusting for tumour stage and grade, indicating that disease severity influences the association.
Factors associated with longer treatment intervals
Factors associated with longer treatment intervals in our study were similar to those reported by other authors,30 including symptom‐detected breast cancer, living in remote areas, and not having private health insurance. Other influential factors included type of screening facility (public or private), time of year when treatment was undertaken, and family history of breast or ovarian cancer. Our sensitivity analysis suggested that the confounding effects of socio‐demographic factors on the association between treatment intervals and survival was minimal.
Limitations
Chemotherapy and radiotherapy treatment dates were retrospectively identified during the telephone interviews, but inaccuracies in the reported dates would probably have reduced estimated associations between treatment intervals and survival; that is, the effects of treatment intervals on survival may have been more marked than we report. Further, reported dates of surgery (94% of breast‐conserving surgery, 87% of mastectomies) was checked against clinical records (Pearson r = 0.99). As the recommended treatment timeframes were published in 2020, about seven years after the most recent diagnoses of breast cancer in women included in our cohort, any change in the proportion of women for whom treatment intervals were longer than recommended is unlikely to have affected our survival analysis.
Conclusion
Breast cancer‐specific survival was poorer for women with breast cancer for whom the diagnosis to surgery, surgery to chemotherapy, or chemotherapy to radiotherapy intervals exceeded Australian guideline‐recommended limits than for women who received more timely care. Our findings provide support for the 2020 treatment guidelines and their recommended timeframes.
Box 1 – Cancer Australia treatment guidelines: recommended treatment timeframes6
|
Interval |
Guideline recommendation |
Level of evidence* |
|||||||||||||
|
|
|||||||||||||||
|
1. Diagnosis to neoadjuvant therapy |
Neoadjuvant systemic therapy should start as soon as diagnosis and staging are completed (ideally within 2–4 weeks) |
Level V, grade A (ESMO 2019 guidelines7) |
|||||||||||||
|
2. Neoadjuvant therapy to surgery |
Perform surgery within 4–6 weeks of neoadjuvant systemic therapy, allowing for recovery from myelosuppression |
Expert consensus (no evidence‐based recommendation identified) |
|||||||||||||
|
3. Diagnosis to surgery |
Perform surgery within one month of decision to treat with surgery women who do not receive neoadjuvant therapy |
Expert consensus (no evidence‐based recommendation identified) |
|||||||||||||
|
4. Surgery to chemotherapy |
Adjuvant chemotherapy should commence within 4–6 weeks of surgery |
1. Level I, grade A (ESMO 2019 guidelines8) |
|||||||||||||
|
5. Surgery to radiotherapy |
Women who have completed definitive surgery for breast cancer should commence radiotherapy as soon as possible after wound healing, and within eight weeks of surgery if no adjuvant chemotherapy received |
Expert consensus (no evidence‐based recommendation identified) |
|||||||||||||
|
6. Chemotherapy to radiotherapy |
Women who have completed definitive surgery for breast cancer should commence radiotherapy within 3–4 weeks of completing adjuvant chemotherapy |
Expert consensus (no evidence‐based recommendation identified) |
|||||||||||||
|
|
|||||||||||||||
|
ESMO = European Society for Medical Oncology; RACP = Royal Australasian College of Physicians. * ESMO: Infectious Diseases Society of America–United States Public Health Service system; RACP: National Health and Medical Research Council system (Supporting Information, box 1). |
|||||||||||||||
Box 2 – Overall non‐compliance (red) and compliance (blue) with Cancer Australia guideline‐recommended treatment timeframes,6 by characteristics of women*
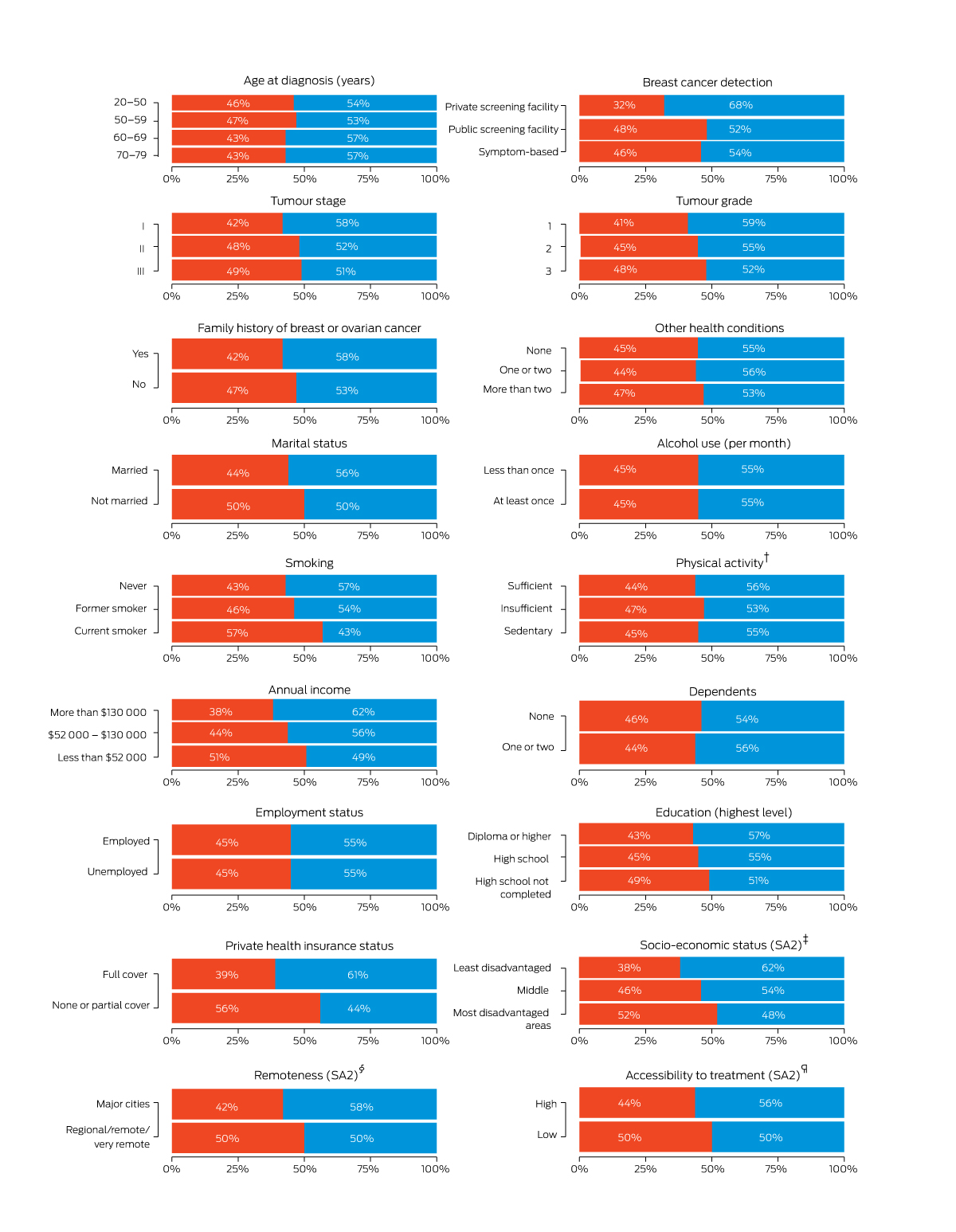
SA2 = Statistical Area level 2.18The data for this figure are included in the Supporting Information, table 2.† Based on weekly activity score before diagnosis, comprising walking per week (minutes) + moderate activity per week (minutes) + 2 × vigorous activity per week (minutes); 0 = sedentary, 1–149 = insufficient, ≥ 150 = sufficient).‡ Index of Relative Socio‐economic Advantage and Disadvantage.19 § Australian Statistical Geography Standard.20 ¶ Based on the road travel time from the residential SA2 to the closest radiation facility (high: less than one hour; low: one hour or more).
Box 3 – Non‐compliance with Cancer Australia guideline‐recommended treatment timeframes6 for 3044 women women aged 20–79 years diagnosed with invasive breast cancer in Queensland, 1 March 2010 – 30 June 2013
|
|
|
|
Delay beyond recommendation (days) |
||||||||||||
|
Intervals |
Eligible women |
Guideline non‐compliance |
Median (IQR) |
Range |
|||||||||||
|
|
|||||||||||||||
|
1. Diagnosis to neoadjuvant therapy |
Received neoadjuvant chemotherapy: 67 |
More than 28 days: 17 (25%) |
12 (6–19) |
1–71 |
|||||||||||
|
2. Neoadjuvant therapy to surgery |
Received neoadjuvant chemotherapy: 67 |
More than 42 days: 7 (10%) |
16 (5–33) |
2–71 |
|||||||||||
|
3. Diagnosis to surgery |
Underwent surgery/did not receive neoadjuvant therapy: 2972 |
More than 45 days:* 135 (4.5%) |
10 (4–20) |
1–428 |
|||||||||||
|
4. Surgery to chemotherapy |
Underwent surgery/received adjuvant chemotherapy: 1574 |
More than 42 days: 464 (29%) |
11 (5–21) |
1–151 |
|||||||||||
|
5. Surgery to radiotherapy |
Underwent surgery/received adjuvant radiotherapy/did not receive adjuvant chemotherapy: 1071 |
More than 56 days: 525 (49%) |
17 (7–30) |
1–304 |
|||||||||||
|
6. Chemotherapy to radiotherapy |
Underwent surgery/received adjuvant chemotherapy/received radiotherapy after completion of chemotherapy: 1156 |
More than 28 days: 443 (38%) |
11 (5–21) |
1–117 |
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Modified from recommendation because date of decision to treat unavailable. |
|||||||||||||||
Box 4 – Comparison of survival for women in the overall non‐compliance and compliance groups: survival differences (guideline compliance v non‐compliance) and hazard ratios* (with 95% confidence intervals, shaded)
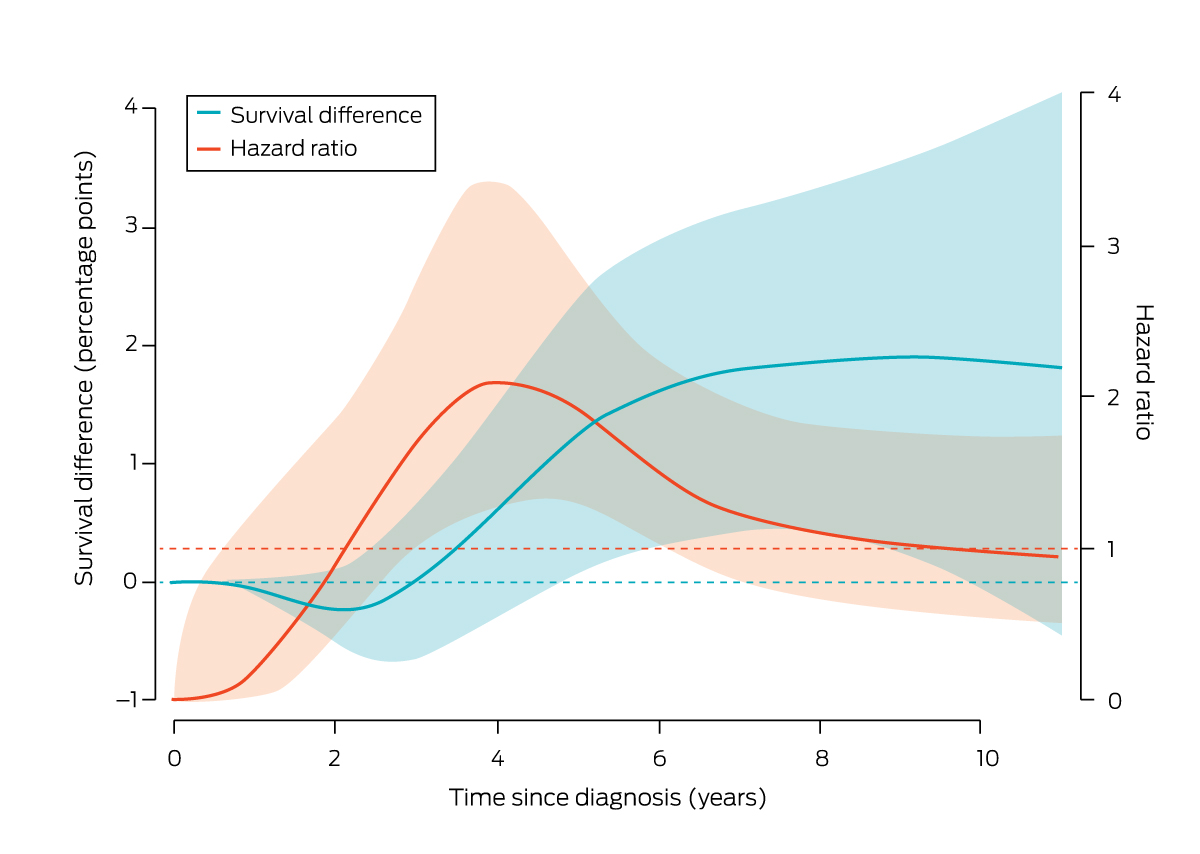
* Reference: hazard for women “guideline compliance” group.
Box 5 – Comparison of survival for women in the overall non‐compliance and compliance groups: survival differences (guideline compliance v non‐compliance), by treatment interval (intervals 3 to 6)*
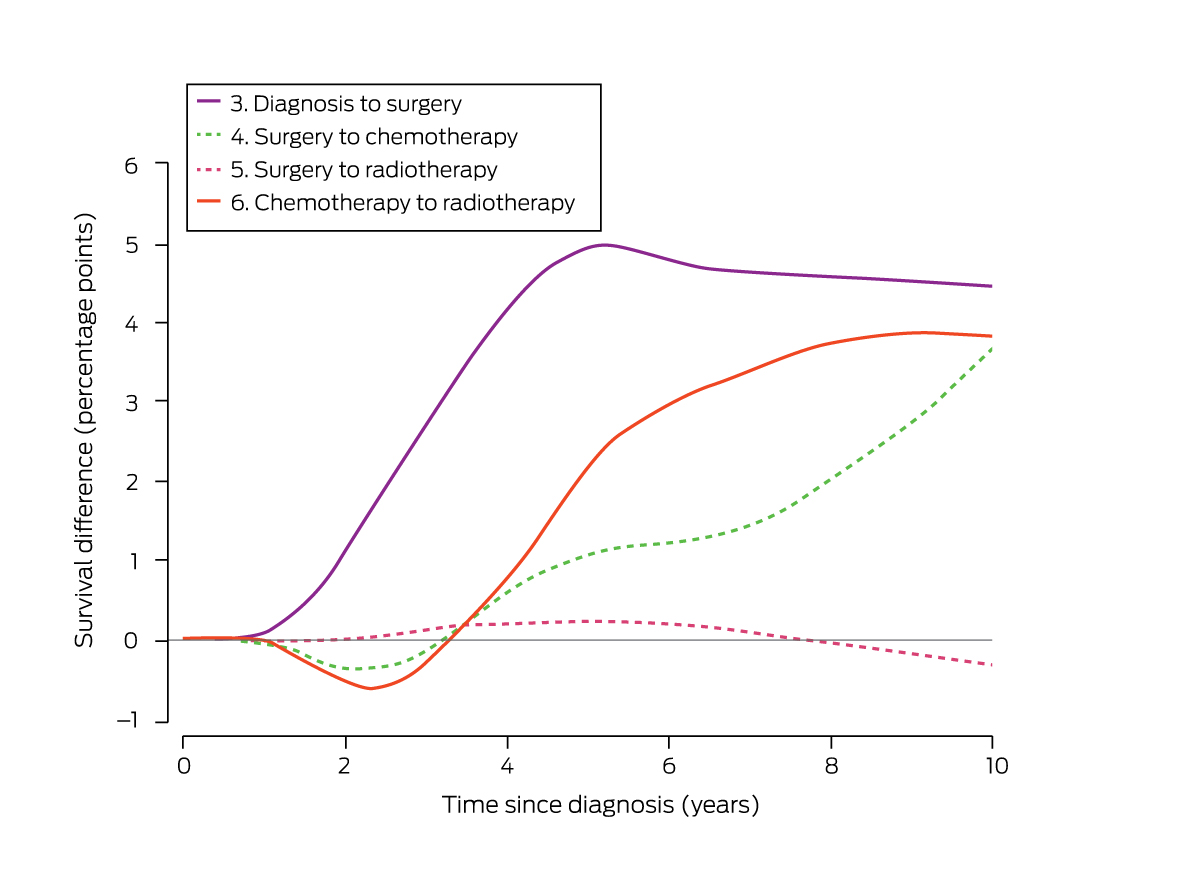
* Statistically significant survival differences are indicated by solid curves, non‐significant differences by dash curves. The survival difference curves for intervals 1 and 2 (the small number of eligible women led to broad 95% confidence intervals requiring a broader y‐axis scale) are included in the Supporting Information, figure 3.
Box 6 – Estimated optimal cut‐points for early breast cancer treatment intervals 3 to 6
|
Interval and guideline‐recommended limit6 |
Optimal cut‐point† |
Adjusted hazard ratio‡ (95% CI) |
Women treated |
||||||||||||
|
Before cut‐point |
After cut‐point |
||||||||||||||
|
|
|||||||||||||||
|
3. Diagnosis to surgery: within one month* |
29 days from diagnosis |
1.76 (1.19–2.59) |
2492 (84%) |
480 (16%) |
|||||||||||
|
4. Surgery to chemotherapy: within 4–6 weeks |
36 days from surgery |
1.63 (1.13–2.36) |
878 (56%) |
696 (44%) |
|||||||||||
|
5. Surgery to radiotherapy: within eight weeks of surgery if no adjuvant chemotherapy received |
No optimal cut‐point (to 103 days) |
1.00 (0.98–1.01) |
— |
— |
|||||||||||
|
6. Chemotherapy to radiotherapy: within 3–4 weeks |
31 days from completion of adjuvant chemotherapy |
1.83 (1.19–2.80) |
781 (68%) |
375 (32%) |
|||||||||||
|
|
|||||||||||||||
|
CI = 95% confidence interval. * Modified to surgery within 45 days after diagnosis, assuming fifteen days for decision making. † The candidate cut‐points are included in the Supporting Information, table 4; the survival curves are depicted in the Supporting Information, figure 4. ‡ Treated after cut‐point v treated before cut‐point, adjusted for age at diagnosis, tumour stage and grade, and mode of detection. |
|||||||||||||||
Box 7 – Characteristics associated with overall guideline non‐compliance: logistic regression analysis*
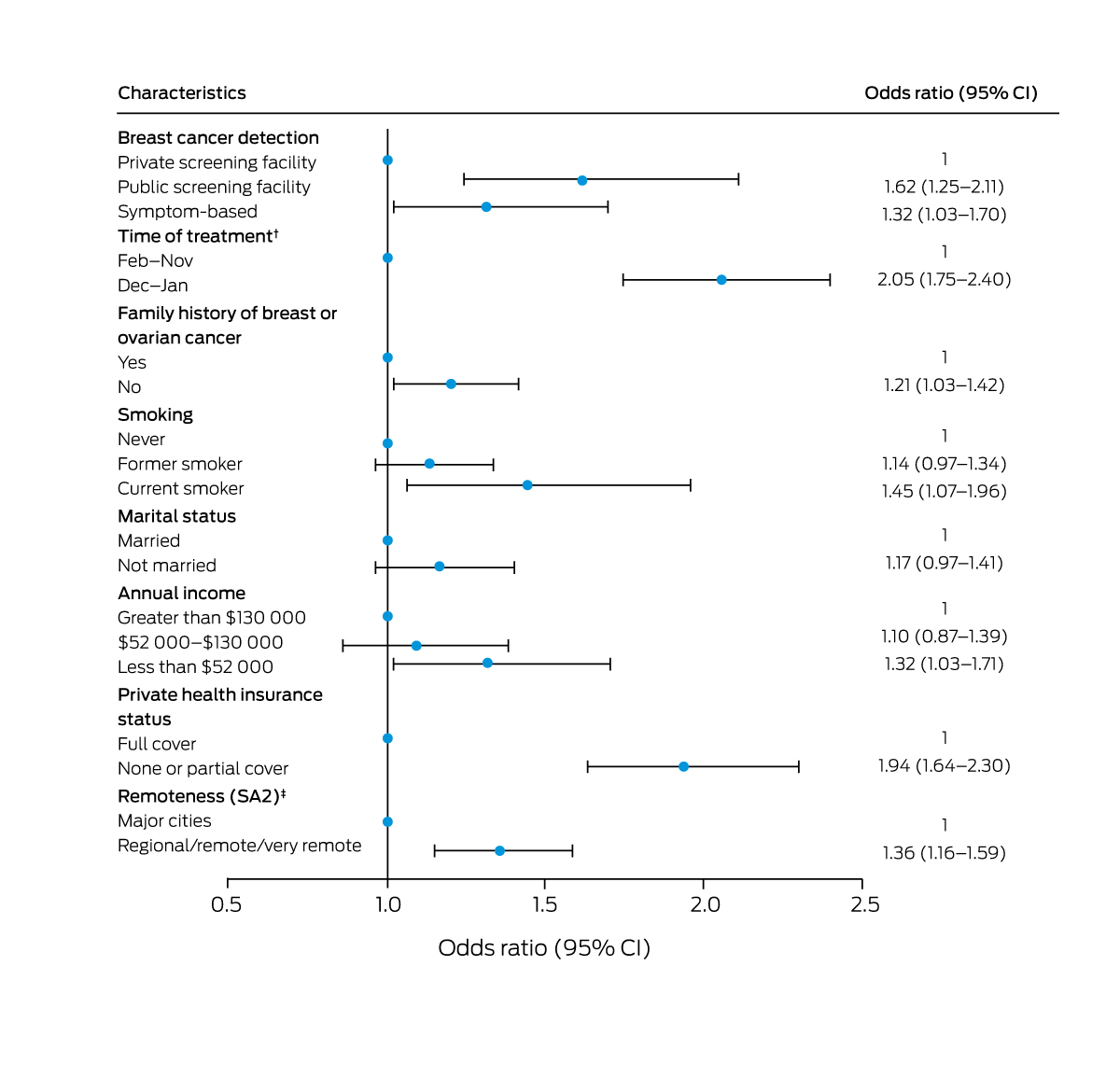
CI = confidence interval; SA2 = Statistical Area level 2.18 * Models adjusted for the number of treatments.† Completion date of the earlier treatment and commencement date of the later treatment for each treatment interval.‡ Australian Statistical Geography Standard.20
Received 3 April 2023, accepted 18 July 2023
- Kou Kou1
- Joanne F Aitken1
- Christopher Pyke2
- Suzanne Chambers3
- Jeff Dunn4
- Peter D Baade1,5
- 1 Viertel Cancer Research Centre, Cancer Council Queensland, Brisbane, QLD
- 2 The University of Queensland, Brisbane, QLD
- 3 University of Technology Sydney, Sydney, NSW
- 4 Prostate Cancer Foundation of Australia, Sydney, NSW
- 5 Queensland University of Technology, Brisbane, QLD
Open access:
Open access publishing facilitated by Queensland University of Technology, as part of the Wiley – Queensland University of Technology agreement via the Council of Australian University Librarians.
This study was funded by a Cancer Australia (100639) and Cancer Council Queensland. We thank the Queensland Cancer Register staff for facilitating data linkage.
No relevant disclosures.
- 1. Australian Institution of Health and Welfare. Cancer data in Australia. Updated 4 Oct 2022. https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia/contents/cancer‐mortality‐by‐age‐visualisation (view July 2023).
- 2. Minicozzi P, Van Eycken L, Molinie F, et al; European HR Working Group on breast cancer. Comorbidities, age and period of diagnosis influence treatment and outcomes in early breast cancer. Int J Cancer 2019; 144: 2118‐2127.
- 3. Martinez A, Daubisse‐Marliac L, Lacaze JL, et al; EvaSein Group. Treatment time interval in breast cancer: a population‐based study on the impact of type and number of cancer centres attended. Eur J Cancer Care 2022; 31: e13654.
- 4. Pratt D, Burneikis T, Tu C, Grobmyer S. Time to completion of breast cancer treatment and survival. Ann Surg Oncol 2021; 28: 8600‐8608.
- 5. Queensland Health. Queensland breast cancer quality index. Practice indicators of safe, quality cancer care: public and private hospitals 2010–2019. June 2022. https://cancerallianceqld.health.qld.gov.au/reports/breastreport2022website‐breastreport2022/#tab1 (viewed July 2023).
- 6. Cancer Australia. Guidance for the management of early breast cancer: recommendations and practice points. Sept 2020. https://www.canceraustralia.gov.au/publications‐and‐resources/cancer‐australia‐publications/guidance‐management‐early‐breast‐cancer‐recommendations‐and‐practice‐points (viewed July 2023).
- 7. Cancer Australia. Guidance for the management of early breast cancer: treatment. Undated. https://www.guidancebreastcancer.gov.au/treatment (viewed July 2023).
- 8. Cardoso F, Kyriakides S, Ohno S, et al; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2019; 30: P1194‐P1220.
- 9. Alexander M, Beattie‐Manning R, Blum R, et al. Guidelines for timely initiation of chemotherapy: a proposed framework for access to medical oncology and haematology cancer clinics and chemotherapy services. Intern Med J 2016; 46: 964‐969.
- 10. Youl PH, Baade PD, Aitken JF, et al. A multilevel investigation of inequalities in clinical and psychosocial outcomes for women after breast cancer. BMC Cancer 2011; 11: 415.
- 11. Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009; 9: 265‐290.
- 12. Baade PD, Fowler H, Kou K, et al. A prognostic survival model for women diagnosed with invasive breast cancer in Queensland, Australia. Breast Cancer Res Treat 2022; 195: 191‐200.
- 13. Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6: 461‐464.
- 14. Williams BA, Mandrekar JN, Mandrekar SJ, et al. Finding optimal cutpoints for continuous covariates with binary and time‐to‐event outcomes (Technical report series #79). Department of Health Sciences Research, June 2006. https://www.mayo.edu/research/documents/biostat‐79pdf/doc‐10027230 (viewed July 2023).
- 15. Derksen S, Keselman HJ. Backward, forward and stepwise automated subset selection algorithms: frequency of obtaining authentic and noise variables. Br J Math Stat Psychol 1992; 45: 265‐282.
- 16. Rubin DB. Multiple imputation for survey nonresponse. New York: Wiley, 1987.
- 17. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011; 30: 377‐399.
- 18. Australian Bureau of Statistics. Statistical Area Level 2. Australian Statistical Geography Standard (ASGS) edition 3; reference period July 2021 – June 2026. 20 July 2021. https://www.abs.gov.au/statistics/standards/australian‐statistical‐geography‐standard‐asgs‐edition‐3/jul2021‐jun2026/main‐structure‐and‐greater‐capital‐city‐statistical‐areas/statistical‐area‐level‐2 (viewed July 2023).
- 19. Australian Bureau of Statistics. Census of Population and Housing: Socio‐Economic Indexes for Areas (SEIFA), 2016 (2033.0.55.001): IRSAD. 27 Mar 2018. https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2016~Main%20Features~IRSAD~20 (viewed July 2023).
- 20. Australian Bureau of Statistics. Australian Statistical Geography Standard (ASGS), volume 5: remoteness structure, July 2016 (1270.0.55.005). 16 Mar 2018. https://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005 (viewed July 2023).
- 21. Mateo AM, Mazor AM, Obeid E, et al. Time to surgery and the impact of delay in the non‐neoadjuvant setting on triple‐negative breast cancers and other phenotypes. Ann Surg Oncol 2020; 27: 1679‐1692.
- 22. Eriksson L, Bergh J, Humphreys K, et al. Time from breast cancer diagnosis to therapeutic surgery and breast cancer prognosis: A population‐based cohort study. Int J Cancer 2018; 143: 1093‐1104.
- 23. Dunn J, Koczwara B, Chambers S. Rethinking cancer survivorship: the Prostate Cancer Survivorship Essentials Framework. Med J Aust 2021; 215: 94. https://www.mja.com.au/journal/2021/215/2/rethinking‐cancer‐survivorship‐prostate‐cancer‐survivorship‐essentials‐framework
- 24. Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys 2006; 65: 1353‐1360.
- 25. Vujovic O, Yu E, Cherian A, et al. Eleven‐year follow‐up results in the delay of breast irradiation after conservative breast surgery in node‐negative breast cancer patients. Int J Radiat Oncol Biol Phys 2006; 64: 760‐764.
- 26. Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast‐conserving surgery to radiation therapy are associated with inferior outcome for women with early‐stage breast cancer who are not receiving chemotherapy. J Clin Oncol 2009; 27: 16‐23.
- 27. Bleicher RJ, Meena SM, Ruth K, et al. The impact of radiotherapy delay in breast conservation patients not receiving chemotherapy and the rationale for dichotomizing the radiation oncology time‐dependent standard into two quality measures. Ann Surg Oncol 2022; 29: 469‐481.
- 28. Cao L, Xu C, Cai G, et al. How does the interval between completion of adjuvant chemotherapy and initiation of radiotherapy impact clinical outcomes in operable breast cancer patients? Ann Surg Oncol 2021; 28: 2155‐2168.
- 29. Chen SY, Sun GY, Tang Y, et al. Timing of postmastectomy radiotherapy following adjuvant chemotherapy for high‐risk breast cancer: a post hoc analysis of a randomised controlled clinical trial. Eur J Cancer 2022; 174: 153‐164.
- 30. Ruco A, Groome PA, McBride ML, et al. Factors associated with the breast cancer diagnostic interval across five Canadian provinces: a CanIMPACT retrospective cohort study. Cancers (Basel) 2023; 15: 404.






Abstract
Objectives: To assess associations between breast cancer‐specific survival and timeliness of treatment, based on 2020 Australian guidelines for the treatment of early breast cancer.
Design: Population‐based cohort study; analysis of linked Queensland Cancer Register, patient medical record, and National Death Index data, supplemented by telephone interviews.
Setting, participants: Women aged 20–79 years diagnosed with invasive breast cancer during 1 March 2010 – 30 June 2013, followed to 31 December 2020.
Main outcome measures: Breast cancer‐specific survival for women who received or did not receive treatment within the recommended timeframe, overall and for six treatment intervals; optimal cut‐points for each treatment interval; characteristics of women for whom treatment was not provided within the recommended timeframe.
Results: Of 5426 eligible women, 4762 could be invited for interviews; complete data were available for 3044 women (56% of eligible women, 65% of invited women). Incomplete compliance with guideline interval recommendations was identified for 1375 women (45%); their risk of death from breast cancer during the follow‐up period was greater than for those for whom guideline compliance was complete (adjusted hazard ratio [aHR], 1.43; 95% confidence interval [CI], 1.04–1.96). Risk of death was greater for women for whom the diagnosis to surgery interval exceeded 29 days (aHR, 1.76; 95% CI, 1.19–2.59), the surgery to chemotherapy interval exceeded 36 days (aHR, 1.63; 95% CI, 1.13–2.36), or the chemotherapy to radiotherapy interval exceeded 31 days (aHR, 1.83; 95% CI, 1.19–2.80). Treatment intervals longer than recommended were more frequent for women for whom breast cancer was detected by public facility screening (adjusted odds ratio [aOR], 1.58; 95% CI, 1.22–2.04) or by symptoms (aOR, 1.39; 95% CI, 1.09–1.79) than when cancer had been detected in private facilities, and for women without private health insurance (aOR, 1.96; 95% CI, 1.66–2.32) or living outside major cities (aOR, 1.38; 95% CI, 1.18–1.62).
Conclusions: Breast cancer‐specific survival was poorer for women for whom the diagnosis to surgery, surgery to chemotherapy, or chemotherapy to radiotherapy intervals exceeded guideline‐recommended limits. Our findings support 2020 Australian guideline recommendations regarding timely care.