The known: People with suspected coronary artery disease (CAD) in rural or remote Australia are often referred to metropolitan hospitals for invasive coronary angiography (ICA), incurring substantial costs and inconvenience.
The new: Of 1017 people from rural or remote Western Australia referred for ICA in Perth, one‐third had non‐obstructive CAD and two‐thirds did not require revascularisation. Computed tomography coronary angiography (CTCA) in rural centres could identify non‐obstructive CAD and consequently avert 53% of referrals and reduce health care costs by $7.3 million (36%).
The implications: Providing CTCA in rural centres could improve access to cardiac services and reduce the costs associated with sending people for ICA in Perth.
Coronary artery disease (CAD) is a leading cause of morbidity and mortality.1 The burden is greater and patient outcomes are poorer in rural and remote Australia than in metropolitan areas.2 One important factor is the limited access to specialist cardiac care in less populated regions. Western Australia is characterised by large distances between its rural centres and metropolitan hospitals. People with suspected CAD in rural or remote areas are often transferred by air to metropolitan hospitals for cardiac investigations such as invasive coronary angiography (ICA), an approach associated with substantial costs, procedure‐related complications, and inconvenience for the patient.3,4 As many people who undergo ICA do not have obstructive CAD or need revascularisation, alternative, non‐invasive risk stratification strategies are needed.5
Computed tomography coronary angiography (CTCA) is a non‐invasive imaging modality for comprehensively evaluating the burden and severity of CAD.6 CTCA can detect high risk plaque and assess lesion‐specific ischaemia using the standard acquisition and computed tomography (CT)‐derived fractional flow reserve (CT‐FFR) respectively.6,7 Making CTCA available in rural centres with 64‐slice (or better) CT scanners (, figure 1) would improve access to cardiac services.8 CTCA could also identify people with non‐obstructive CAD who do not require transfer to metropolitan hospitals for ICA. However, the costs of establishing CTCA facilities in rural Australia have not been evaluated.6
We investigated the severity of CAD in people from rural or remote Western Australia referred for ICA in Perth and their subsequent management. We also estimated the potential cost savings were CTCA available in rural centres for first line investigation of people with suspected CAD.
Methods
We undertook a retrospective cross‐sectional study of adults (18 years or older) referred from rural and remote WA hospitals and primary care centres to the three public tertiary hospitals in Perth (Fiona Stanley, Royal Perth, Sir Charles Gairdner Hospitals) for ICA evaluation of suspected CAD during the 2019 calendar year. This was the most recent year in which hospital resource levels and clinical decision making were not influenced by the coronavirus disease 2019 (COVID‐19) pandemic. “Rural” and “remote” were defined according to the Modified Monash Model.9 We extracted patient data from electronic medical records, including discharge summaries and the central angiography database for all public hospitals in WA. We excluded people with unstable presentations (ST‐elevation myocardial infarction, cardiac arrest, cardiogenic shock, unstable arrhythmia), as well as people who were referred for elective revascularisation, had been evaluated for CAD during the preceding three months, or for whom data were incomplete. We classified indications for ICA as non‐ST elevation myocardial infarction (NSTEMI), chest pain with normal troponin levels, or other (assessment of valvular heart disease, arrhythmia, or cardiomyopathy).
Coronary artery disease: characteristics and management
Severity of CAD was derived from information in ICA reports. Stenosis severity was defined according to the coronary artery disease reporting and data system (CAD‐RADS), based on the most severe stenosis detected: no stenosis (0%), minimal (1–24%), mild (25–49%), moderate (50–69%), severe (70–99%, or > 50% in left main coronary artery), and occluded (100%).10 Management was categorised as medical or revascularisation (percutaneous coronary intervention or coronary artery bypass graft surgery). The efficiency of ICA resource use was defined by the ICA:revascularisation ratio.11 We assumed 99% sensitivity and 88% specificity for CTCA detecting obstructive CAD (≥ 50% stenosis), based upon a subset of patients in a meta‐analysis in which ICA was the reference assessment method.12
Health care costs
The three health care system cost components for the year 2019 we assessed (, table 1) were:- the cost of transfer from a rural or remote location to a metropolitan hospital;
- the cost of the metropolitan hospital admission, based on length of stay; and
- the cost of ICA with or without revascularisation.
For inter‐hospital transfers, Royal Flying Doctor Service costs were obtained from the WA Country Health Service. For elective transfers, the cost was calculated as the maximum patient subsidy provided by the WA Country Health Service for self‐funded travel according to location and distance. Costs of the hospital admission, ICA, and revascularisation were estimated using diagnosis‐related groups (DRG) codes obtained from the WA Health Business Team.
Based on the Medicare Benefits Schedule (2019), the cost of CTCA was estimated to be $716.90 (item 57360) and of stress echocardiography $656.85 (items 55132, 55143). The time of lost productivity was deemed to be equivalent to the length of the metropolitan hospital stay, and income lost for people of working age (15–64 years) was calculated from Australian Bureau of Statistics age‐ and sex‐adjusted incomes data for 2018–19 (, table 2).13
Alternative care model: local computed tomography coronary angiography
We also examined a potential alternative model of care for which we assumed that CTCA could be performed in rural centres (Box 1). People with normal or non‐obstructive CAD (< 50% stenosis) according to CTCA would not require ICA and consequently not be transferred to metropolitan hospitals, but those with severe CAD (≥ 70% stenosis) would be transferred. People with moderate CAD (50–69% stenosis) would undergo functional testing (stress echocardiography or CT‐FFR) in the rural centre; for the functional testing outcome we used the actual revascularisation outcome. We compared health care costs for usual care (ie, as determined by retrospective review of medical records) and for the alternative care model (simulated rural CTCA results based on individuals’ actual ICA findings).
Statistical analysis
Analyses were performed in SAS 9.4. We summarise data as means with standard deviations (SDs), numbers and proportions, or medians with interquartile ranges (IQRs). Associations of CAD severity and treatment with indication for ICA were assessed using binary logistic regression analysis and reported as odds ratio (ORs) with 95% confidence interval (CIs). P < 0.05 was deemed statistically significant.
Ethics approval
This study was approved as a clinical audit by the South Metropolitan Health Service (Western Australia) Governance, Evidence, Knowledge and Outcomes committee (FSFHG GEKO42539).
Results
Of 1596 people from rural or remote WA referred for ICA in Perth during 2019, 508 were ineligible for our study and 71 did not undergo ICA (Box 2). The mean age of the 1017 people who underwent ICA was 62 years (SD, 13 years), 680 were men (66.9%), and 245 were Indigenous people (24.1%) (Box 3). A total of 411 people were referred for ICA as elective outpatients (40.4%). Of the 606 people transferred between regional and Perth hospitals (inter‐hospital transfers; 59.6%), the indication was NSTEMI in 425 cases (70.1%) and chest pain with normal troponin levels in 132 (21.8%).
Coronary artery disease: characteristics
Eighty of the 1017 people who underwent ICA had no coronary artery stenosis (7.9%), 285 had minimal or mild CAD (28.0%), 137 had moderate CAD (13.5%), and 515 had severe CAD or an occluded vessel (50.7%) (Box 4). A larger proportion of people with NSTEMI had obstructive CAD (≥ 50% stenosis) than of those with alternative indications for ICA (327 of 438 [74.7%] v 325 of 579 [56.1%]; OR, 2.3; 95% CI, 1.8–3.0).
Coronary artery disease: management
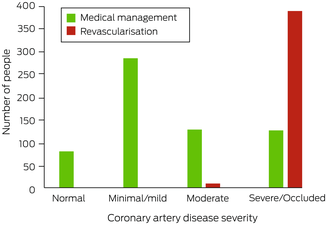
After undergoing ICA, 619 people were medically managed (60.9%) and 398 underwent revascularisation (39.1%; ICA:revascularisation ratio, 2.6). No‐one with non‐obstructive CAD (< 50% stenosis), nine with moderate CAD (7%), and 389 with severe CAD or an occluded vessel (75.5%) underwent revascularisation (Box 5). A larger proportion of people with NSTEMI underwent revascularisation (55%) than of those with chest pain and normal troponin levels (35%; OR, 2.3; 95% CI, 1.7–3.0) or other indications for ICA (10%; OR, 11; 95% CI, 6.4–18) (Box 6). Twenty‐three people experienced peri‐procedural complications (2.3%) (, table 3).
Health care costs
Total estimated health care costs for the 1017 people who underwent ICA in Perth were $20.3 million; substantial costs were associated with aeromedical transfer (data not shown). The estimated costs by indication were $12.4 million for NSTEMI (60.9%), $5.4 million for chest pain with normal troponin level (26.7%), and $2.5 million for other (12.4%) (Box 7). Mean lost productivity for the 612 people of working age was 3.4 days (SD, 3.0 days) and the mean income lost $754.81 (SD, $728.87).
Local computed tomography coronary angiography model of care
Were CTCA undertaken in rural centres, 573 referrals could have been averted (52.7%), the number of metropolitan hospital bed‐days reduced from 4065 to 2308 (43% reduction), and the ICA:revascularisation ratio reduced from 2.6 to 1.6. Total estimated health care costs would be reduced from $20.3 million to $13.0 million (36% reduction) (Box 8). The cost proportion saved was lower for people with NSTEMI as ICA indication ($3.2 million, 26%) than for those with chest pain and normal troponin levels ($2.4 million, 44%) or other indications ($1.6 million, 64%). Not transferring people of working age with non‐severe stenosis would save a mean 3.0 days (SD, 2.6 days) of lost productivity and $619.90 in lost income per patient (SD, $568.00).
Assuming 99% sensitivity and 88% specificity for CTCA detecting obstructive CAD (compared with ICA), obstructive CAD would be determined by CTCA in 44 people with non‐obstructive CAD (4.3% of people assessed with ICA), and obstructive CAD would be missed in seven people with obstructive CAD (0.7%) (, table 4).
Discussion
We report the first study of CAD severity in people in rural and remote locations of WA referred with non‐urgent indications to metropolitan tertiary hospitals for ICA evaluation. More than one‐third of people in the study had normal coronary arteries or non‐obstructive CAD (< 50% stenosis), all of whom were medically managed. About 75% of people with severe CAD or an occluded vessel underwent revascularisation (389 of 515), but only 7% (nine of 137) with moderate CAD (50–69% stenosis); in total, for every 2.6 ICA assessments undertaken, one patient underwent revascularisation. We also found that undertaking CTCA in rural centres could avert 53% of transfers for ICA by locally identifying people with non‐obstructive CAD who may not require ICA. This would reduce the number of required metropolitan hospital bed‐days by 43%, health care costs by 36%, the ICA:revascularisation ratio from 2.6 to 1.6, and lost productivity by three days per patient.
As transferring people from rural and remote areas to metropolitan hospitals is expensive — a large proportion of the total costs are for aeromedical retrieval — unnecessary transfers should be avoided. Not unexpectedly, the costs of inter‐hospital transfers of people with NSTEMI accounted for two‐thirds of all health care costs. Although a larger proportion of patients with NSTEMI (55%) than of people with alternative indications for ICA underwent revascularisation, almost half were medically managed. To improve resource use, reduce the risk of ICA‐related complications, and minimise inconvenience for patients, it is therefore desirable to locally identify people with non‐obstructive CAD, who may not require revascularisation.
CTCA is an established non‐invasive modality recommended in guidelines as a first line investigation for people with stable CAD.14,15 CTCA may also be useful for assessing people with NSTEMI at low to intermediate risk of cardiovascular events, given its high diagnostic accuracy for ruling out clinically significant CAD, and accuracy equivalent to that of ICA for assessing long term risk.16,17 Although we included people with NSTEMI, CTCA is not considered a gatekeeper assessment for ICA in such cases. Local CTCA may not be appropriate for all people presenting with NSTEMI, particularly those at high risk of cardiovascular events. Indeed, we found that the cost savings were lower when NSTEMI was the indication for ICA, as obstructive CAD and the need for revascularisation were more likely than with alternative indications. The DISCHARGE trial found that the risk of cardiovascular events in people with stable symptoms and intermediate probability of obstructive CAD referred for ICA was similar whether they underwent CTCA or ICA first; however, a smaller proportion of people who underwent CTCA first experienced major procedural complications (0.5% v 1.9%).18
One advantage of CTCA is that it can detect mild CAD, enabling clinicians to initiate or intensify preventive therapies. Non‐obstructive CAD is an important substrate for plaque rupture and myocardial infarction not detected by functional testing. The SCOT‐HEART trial found that CTCA was associated with better coronary heart disease outcomes for people with stable chest pain, probably because of earlier anatomic characterisation and initiation of medical therapies (eg, aspirin, statins), regardless of CAD stage.19
Given the challenges of providing cardiac services in rural and remote areas,8,20,21 we support offering CTCA in rural WA to improve access to cardiac care and reduce health care costs. Further, transfers to metropolitan hospitals cause lost productivity and inconvenience for patients. Indigenous people, 24% of those referred to metropolitan hospitals for ICA during 2019, may be particularly affected by family, cultural, and social disruptions. Investment in CTCA technology, accreditation, and training would be needed to provide CTCA in rural centres.21
High initial costs could be a considerable barrier, and other practical details must also be investigated. On the other hand, reducing the numbers of inter‐hospital transfers and ICA assessments could lead to greater efficiency and substantial long term cost savings. Several factors make CTCA more attractive than other cardiac imaging modalities in rural and remote areas, including the speed of image acquisition, its high volume capacity, the relatively low level of ionising radiation, and the ability to identify non‐obstructive CAD. CTCA can also provide information about high risk plaque, peri‐coronary inflammation, cardiac chamber size, and diastolic function.6,7,22
Determining the functional significance of moderate stenosis detected by CTCA can be difficult. Functional testing is typically undertaken to determine the haemodynamic significance of a moderate stenosis and whether ICA is indicated, but such testing is logistically challenging in rural areas, as it requires specific expertise and access to yet another testing modality. Lesion‐specific ischaemia can be determined by applying CT‐FFR to the standard CTCA acquisition (without additional radiation exposure or medications), and anatomically significant stenoses could be re‐classified as functionally non‐significant, thereby averting further functional testing and ICA (Box 9).18 CT‐FFR is not used clinically in Australia. but is commercially available in the United States, the United Kingdom, and Japan; in the United States, it is recommended for assessing people with stable, acute chest pain.15 CT‐FFR could enhance the value of CTCA in rural areas, as its diagnostic performance is superior to that of other functional tests, it reduces the need for ICA, and it predicts patient outcomes.23,24,25
Limitations
The retrospective nature of our study and its estimation of costs on the basis of DRG codes were limitations. The general application of CTCA (as not all contraindications could be identified) was also suboptimal. Although false positive CTCA findings could lead to potentially unnecessary transfers, the proportion of people affected is probably small (4.3% of people in our study) and health care cost savings would still be substantial were they transferred. Moreover, the impact of false positives would be reduced by the addition of functional testing in rural areas for people with moderate stenoses, as proposed in our care model.12 Our estimated costs for CTCA in rural areas did not include costs for upgrading scanner software for 64‐slice CT scanners, maintaining equipment, or training radiographers and expert readers, as accurate estimates could not be obtained for each rural site. Conversely, savings would be higher were the costs of patients waiting in rural centres for transfer and the impact on metropolitan hospital bed flow for avoidable admissions taken into account.
Conclusion
About one‐third of people in rural and remote WA referred for ICA evaluation of CAD during 2019 had non‐obstructive CAD, and almost two‐thirds were medically managed. By identifying people who may not require ICA, undertaking CTCA in rural centres could avert 53% of referrals to metropolitan hospitals, reduce the number of metropolitan hospital bed‐days by 42%, reduce health care costs by 36% ($7.27 million saved), reduce the proportion of ICA assessments that do not lead to revascularisation, and save three days of lost productivity per patient. The cost‐effectiveness and clinical impact of this model of care should be prospectively evaluated.
Box 1 – Alternative care model: local computed tomography coronary angiography for people in rural or remote areas of Western Australia
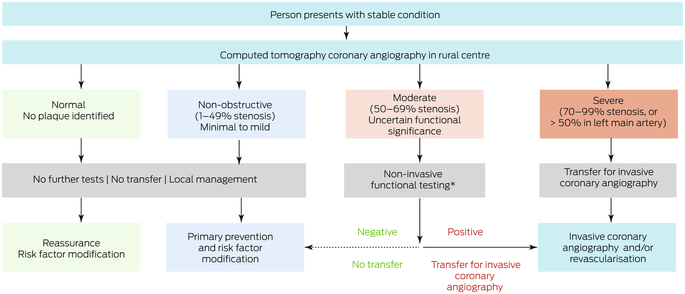
* Stress echocardiography or computed tomography‐derived fractional flow reserve (CT‐FFR).
Box 2 – People in rural or remote Western Australia assessed for cardiac condtions during 2019: selection for inclusion in our study
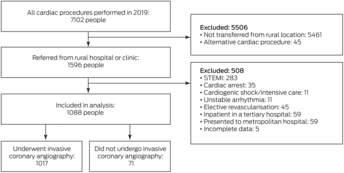
STEMI = ST‐segment elevation myocardial infarction.
Box 3 – Baseline characteristics of 1017 people from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019*
Characteristic |
Number | ||||||||||||||
Total number of people |
1017 |
||||||||||||||
Age (years), mean (SD) |
62 (13) |
||||||||||||||
Sex (men) |
680 (66.9%) |
||||||||||||||
Indigenous people |
245 (24.1%) |
||||||||||||||
Inter‐hospital transfers |
606 (59.6%) |
||||||||||||||
Indication for invasive coronary angiography |
|||||||||||||||
NSTEMI |
438 (43.1%) |
||||||||||||||
Chest pain with normal troponin level |
394 (38.7%) |
||||||||||||||
Valvular heart disease |
76 (7.5%) |
||||||||||||||
Pre‐operative assessment |
27 (2.6%) |
||||||||||||||
Arrhythmia |
23 (2.3%) |
||||||||||||||
Cardiomyopathy |
59 (5.8%) |
||||||||||||||
NSTEMI = non‐ST segment elevation myocardial infarction; SD = standard deviation. * For referrals by region, see , figure 2. | |||||||||||||||
Box 4 – Severity of coronary artery disease in 1017 people from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019, by indication
Coronary artery disease severity (stenosis) | |||||||||||||||
Indication for invasive coronary angiography |
Number of people |
Normal |
Minimal/mild (< 50%) |
Moderate (50–69%) |
Severe (70–99%) |
Occluded (100%) | |||||||||
All indications |
1017 |
80 (7.9%) |
285 (28.0%) |
137 (13.5%) |
378 (37.2%) |
137 (13.5%) |
|||||||||
NSTEMI |
438 |
27 (6.2%) |
84 (19%) |
35 (8.0%) |
218 (49.8%) |
74 (17%) |
|||||||||
Chest pain with normal troponin level |
394 |
25 (6.3%) |
126 (32.0%) |
69 (18%) |
134 (34.0%) |
40 (10%) |
|||||||||
Other* |
185 |
28 (15%) |
75 (40%) |
33 (18%) |
26 (14%) |
23 (12%) |
|||||||||
NSTEMI = non‐ST segment elevation myocardial infarction. * Valvular heart disease, pre‐operative assessment, arrhythmia, cardiomyopathy. | |||||||||||||||
Box 5 – Management of 1017 people from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019, by severity of coronary artery disease
Box 6 – Management outcomes of 1017 people from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019, by indication for referral
Treatment | |||||||||||||||
Indication for invasive coronary angiography |
Medical management |
Revascularisation | |||||||||||||
NSTEMI |
197 (45.0%) |
241 (55.0%) |
|||||||||||||
Chest pain with normal troponin level |
256 (65.0%) |
138 (35.0%) |
|||||||||||||
Other* |
166 (89.7%) |
19 (10%) |
|||||||||||||
NSTEMI = non‐ST segment elevation myocardial infarction. * Valvular heart disease, pre‐operative assessment, arrhythmia, cardiomyopathy. | |||||||||||||||
Box 7 – Total health care costs for 1017 patients from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019, by care model
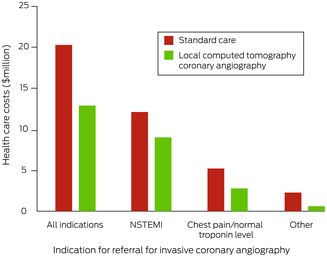
NSTEMI = non‐ST segment elevation myocardial infarction.
Box 8 – Total health care costs for 1017 patients from rural or remote Western Australia who underwent invasive coronary angiography in Perth during 2019, by indication
Care model | |||||||||||||||
Indication for invasive coronary angiography |
Standard care |
Alternative model |
Difference | ||||||||||||
All indications |
$20.31 million |
$13.04 million |
–$7.27 million (35.8%) |
||||||||||||
NSTEMI |
$12.36 million |
$9.13 million |
–$3.24 million (26.2%) |
||||||||||||
Chest pain with normal troponin level |
$5.42 million |
$3.02 million |
–$2.41 million (44.4%) |
||||||||||||
Other* |
$2.52 million |
$0.90 million |
–$1.62 million (64.3%) |
||||||||||||
NSTEMI = non‐ST segment elevation myocardial infarction. * Valvular heart disease, pre‐operative assessment, arrhythmia, cardiomyopathy. | |||||||||||||||
Box 9 – Computed tomography coronary angiography (CTCA) images (A–C) and computed tomography‐derived fractional flow reserve (CT‐FFR) assessment of stenosis (D)*
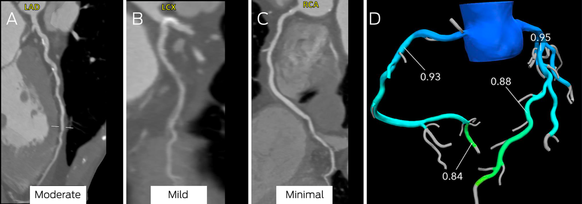
LAD = left anterior descending artery; LCX = left circumflex; RCA = right coronary artery.
Moderate stenosis is evident in the mid‐left anterior descending coronary artery (A), mild disease in the left circumflex coronary artery (B), and minimal disease in the right coronary artery (C). There was no evidence of ischaemia in the left anterior descending coronary artery (D; CT‐FFR, 0.88; normal > 0.80).
Received 4 February 2023, accepted 10 May 2023
- Mikhail Alexander1,2
- Nick S R Lan1,3
- Michael J Dallo1
- Tom G Briffa3
- Frank M Sanfilippo3
- Andrew Hooper4
- Helen Bartholomew4
- Loletta Hii1
- Graham S Hillis2,3
- Brendan M McQuillan3,5
- Girish Dwivedi1,6
- James M Rankin1
- Abdul Rahman Ihdayhid1,6,7
- 1 Fiona Stanley Hospital, Perth, WA
- 2 Royal Perth Hospital, Perth, WA
- 3 The University of Western Australia, Perth, WA
- 4 Medical Royal Flying Doctor Service Western Australia, Perth, WA
- 5 Sir Charles Gairdner Hospital, Perth, WA
- 6 Harry Perkins Institute of Medical Research, Perth, WA
- 7 Curtin Medical School, Curtin University, Perth, WA
Open access:
Open access publishing facilitated by Curtin University, as part of the Wiley – Curtin University agreement via the Council of Australian University Librarians.
Abdul Rahman Ihdayhid is supported by a National Heart Foundation postdoctoral scholarship.
Nick S R Lan has received research funding from Sanofi as part of a clinical fellowship in endocrinology and diabetes, education support from Amgen, Bayer, Boehringer Ingelheim, Eli Lilly, and Novartis, and speaker honoraria from Boehringer Ingelheim, Eli Lilly, and Sanofi, and has participated in advisory boards for Eli Lilly. Girish Dwivedi has received lecture fees from AstraZeneca, Pfizer, and Amgen (not related to the topic of this study), and provides consultancy services and has equity interest in Artrya. Abdul Rahman Ihdayhid is a consultant for Abbott Medical, Boston Scientific, and Artrya (including equity interest).
- 1. Roth GA, Mensah GA, Johnson CO, et al; GBD‐NHLBI‐JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020; 76: 2982‐3021.
- 2. Alston L, Allender S, Peterson K, et al. Rural inequalities in the Australian burden of ischaemic heart disease: a systematic review. Heart Lung Circ 2017; 26: 122‐133.
- 3. Lopez D, Katzenellenbogen JM, Sanfilippo FM, et al. Transfers to metropolitan hospitals and coronary angiography for rural Aboriginal and non‐Aboriginal patients with acute ischaemic heart disease in Western Australia. BMC Cardiovasc Disord 2014; 14: 58.
- 4. Gardiner FW, Rallah‐Baker K, Dos Santos A, et al. Indigenous Australians have a greater prevalence of heart, stroke, and vascular disease, are younger at death, with higher hospitalisation and more aeromedical retrievals from remote regions. EClinicalMedicine 2021; 42: 101181.
- 5. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010; 362: 886‐895.
- 6. Ihdayhid AR, Lan NSR, Figtree GA, et al. Contemporary chest pain evaluation: the Australian case for cardiac CT. Heart Lung Circ 2023; 32: 297‐306.
- 7. Williams MC, Moss AJ, Dweck M, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT‐HEART study. J Am Coll Cardiol 2019; 73: 291‐301.
- 8. Jeffries A, Costello B, Corkill W, et al. Prognostic value of coronary artery calcium scoring and computed tomography coronary angiography in remote Indigenous and non‐Indigenous Australians. Int J Cardiol 2021; 328: 241‐246.
- 9. Australian Department of Health and Aged Care. Modified Monash Model. Updated 14 Dec 2021. https://www.health.gov.au/topics/rural‐health‐workforce/classifications/mmm (viewed Dec 2022).
- 10. Narula J, Chandrashekhar Y, Ahmadi A, et al. SCCT 2021 expert consensus document on coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2021; 15: 192‐217.
- 11. Harper RW, Nasis A, Sundararajan V. How changes to the Medicare Benefits Schedule could improve the practice of cardiology and save taxpayer money. Med J Aust 2015; 203: 256‐258. https://www.mja.com.au/journal/2015/203/6/how‐changes‐medicare‐benefits‐schedule‐could‐improve‐practice‐cardiology‐and
- 12. Nielsen LH, Ortner N, Nørgaard BL, et al. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging 2014; 15: 961‐971.
- 13. Australian Bureau of Statistics. Personal income in Australia. Reference period: 2014–15 to 2018–19. 17 Dec 2021. https://www.abs.gov.au/statistics/labour/earnings‐and‐working‐conditions/personal‐income‐australia/2014‐15‐2018‐19 (viewed Dec 2022).
- 14. Knuuti J, Wijns W, Saraste A, et al; ESC Scientific Document Group. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41: 407‐477.
- 15. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary. A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021; 144: e368‐e454.
- 16. Linde JJ, Kelbæk H, Hansen TF, et al. Coronary CT angiography in patients with non‐ST‐segment elevation acute coronary syndrome. J Am Coll Cardiol 2020; 75: 453‐463.
- 17. Kofoed KF, Engstrøm T, Sigvardsen PE, et al. Prognostic value of coronary CT angiography in patients with non‐ST‐segment elevation acute coronary syndromes. J Am Coll Cardiol 2021; 77: 1044‐1052.
- 18. DISCHARGE Trial Group; Maurovich‐Horvat P, Bosserdt M, Kofoed KF, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med 2022; 386: 1591‐1602.
- 19. SCOT‐HEART Investigators; Newby DE, Adamson PD, Berry C, et al. Coronary CT angiography and 5‐year risk of myocardial infarction. N Engl J Med 2018; 379: 924‐933.
- 20. Kempton HR, Bemand T, Bart NK, Suttie JJ. Using coronary artery calcium scoring as preventative health tool to reduce the high burden of cardiovascular disease in Indigenous Australians. Heart Lung Circ 2020; 29: 835‐839.
- 21. Dreisbach JG, Nicol ED, Roobottom CA, et al. Challenges in delivering computed tomography coronary angiography as the first‐line test for stable chest pain. Heart 2018; 104: 921‐927.
- 22. Lessick J, Mutlak D, Efraim R, et al. Comparison between echocardiography and cardiac computed tomography in the evaluation of diastolic dysfunction and prediction of heart failure. Am J Cardiol 2022; 181: 71‐78.
- 23. Driessen RS, Danad I, Stuijfzand WJ, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 2019; 73: 161‐173.
- 24. Curzen N, Nicholas Z, Stuart B, et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 2021; 42: 3844‐3852.
- 25. Patel MR, Nørgaard BL, Fairbairn TA, et al. 1‐year impact on medical practice and clinical outcomes of FFR(CT): the ADVANCE Registry. JACC Cardiovasc Imaging 2020; 13: 97‐105.






Abstract
Objectives: To examine the severity of coronary artery disease (CAD) in people from rural or remote Western Australia referred for invasive coronary angiography (ICA) in Perth and their subsequent management; to estimate the cost savings were computed tomography coronary angiography (CTCA) offered in rural centres as a first line investigation for people with suspected CAD.
Design: Retrospective cohort study.
Setting, participants: Adults with stable symptoms in rural and remote WA referred to Perth public tertiary hospitals for ICA evaluation during the 2019 calendar year.
Main outcome measures: Severity and management of CAD (medical management or revascularisation); health care costs by care model (standard care or a proposed alternative model with local CTCA assessment).
Results: The mean age of the 1017 people from rural and remote WA who underwent ICA in Perth was 62 years (standard deviation, 13 years); 680 were men (66.9%), 245 were Indigenous people (24.1%). Indications for referral were non‐ST elevation myocardial infarction (438, 43.1%), chest pain with normal troponin level (394, 38.7%), and other (185, 18.2%). After ICA assessment, 619 people were medically managed (60.9%) and 398 underwent revascularisation (39.1%). None of the 365 patients (35.9%) without obstructed coronaries (< 50% stenosis) underwent revascularisation; nine patients with moderate CAD (50–69% stenosis; 7%) and 389 with severe CAD (≥ 70% stenosis or occluded vessel; 75.5%) underwent revascularisation. Were CTCA used locally to determine the need for referral, 527 referrals could have been averted (53%), the ICA:revascularisation ratio would have improved from 2.6 to 1.6, and 1757 metropolitan hospital bed‐days (43% reduction) and $7.3 million in health care costs (36% reduction) would have been saved.
Conclusion: Many rural and remote Western Australians transferred for ICA in Perth have non‐obstructive CAD and are medically managed. Providing CTCA as a first line investigation in rural centres could avert half of these transfers and be a cost‐effective strategy for risk stratification of people with suspected CAD.