Summary box
The known: The World Health Organization aims to eliminate hepatitis C as a public health problem by 2030. Testing and treatment need to increase to meet this target in Australia.
The new: The proportion of people who underwent integrated hepatitis C virus testing, fibrosis assessment, and antiviral therapy at a supervised injecting facility was small, but almost nine in ten people diagnosed with hepatitis C were prescribed direct‐acting antiviral treatment.
The implications: Offering care at supervised injecting facilities could reduce barriers to engagement with care by people at high risk of hepatitis C, and thereby expedite its elimination as a public health threat.
Chronic hepatitis C virus (HCV) infection is associated with substantial morbidity and mortality.1 Injecting drug use is the predominant mode of transmission in developed countries.2 Highly effective and well tolerated direct acting antiviral (DAA) therapies have revolutionised hepatitis C treatment, and the Australian Department of Health aims to eliminate hepatitis C as a public health problem in Australia by 2030, in accordance with World Health Organization targets.3
Engaging people who inject drugs with hepatitis C testing and treatment will be crucial to elimination. Routine hepatitis C testing in community health services attended by people who inject drugs increases their engagement with care.4,5,6,7 Supervised injecting facilities offer an effective, evidence‐based harm reduction strategy that reduces the risks of death from overdose and of blood‐borne virus transmission.8,9 They care for people more likely to engage in high risk injecting behaviours, among whom the prevalence of hepatitis C is higher than in the general community.10,11,12 The effectiveness of hepatitis C programs in supervised injecting facilities has not been investigated in detail.
The first supervised injecting facility in Victoria, opened in 2018 in inner metropolitan Melbourne, can accommodate more than 200 visits per day.13 Since opening, it has offered a comprehensive model of care, incorporating on‐site social and health services, including hepatitis C diagnostic testing, liver fibrosis assessment, and treatment for hepatitis C. We evaluated the proportion of people attending the Melbourne supervised injecting facility tested for hepatitis C during its first two years of operation, and examined factors that influence engagement with testing, the number of people found to have hepatitis C infections, and the number who were prescribed treatment.
Methods
We evaluated the proportion of people who visited the Melbourne supervised injecting facility tested for hepatitis C during 30 June 2018 – 30 June 2020. On‐site hepatitis C care was supported by an outreach hepatitis nursing service based at a tertiary hospital (St Vincent's Hospital, Melbourne). People can use the injecting facility if they are at least 18 years old, not injecting drugs for the first time, not intoxicated, and possess the drugs they intend to inject. Clients are registered, allocated a supervised injecting booth in the injecting zone, then moved to an aftercare zone, where opportunistic blood‐borne virus testing by an on‐site nurse was available 3–5 days a week, 9 am–5 pm (the supervised injecting facility was open for up to 14 hours per day, seven days a week). A protocol‐driven assessment evaluated risk behaviour, previous hepatitis C treatment, self‐reported psychiatric illness, alcohol use, medical conditions, and medication use. Blood was collected for anti‐HCV antibody assay, reflex HCV RNA testing (using the same sample if the person was anti‐HCV antibody‐positive) and genotyping, hepatitis B virus (HBV) and human immunodeficiency virus (HIV) serology, full blood examination, and assessment of alanine transaminase (ALT) and aspartate transaminase (AST) levels. Liver fibrosis was assessed using the AST‐to‐platelet ratio index (APRI);14 people with APRI scores of 1.0 or more were referred for transient elastography (available on site two days a week).
People found to have hepatitis C infections were triaged to simple or complex treatment pathways. The simple pathway was for people with APRI scores below 1.0, who did not have HBV or HIV infections, and for whom no drug–drug interactions were known. They were prescribed DAAs by an on‐site general practitioner after a paper‐based consultation with the hepatitis outreach nurse. The complex pathway was used for people with suspected cirrhosis (APRI score of at least 1.0), HBV or HIV infections, or challenging drug–drug interactions; they were prescribed DAAs by a gastroenterologist after a remote paper‐based consultation or a face‐to‐face clinical assessment. People with suspected cirrhosis or HBV or HIV infections were referred to St Vincent's Hospital, Melbourne for follow‐up by a specialist. DAAs were dispensed from the hospital outpatient pharmacy, which waived the drug co‐payment fee and delivered medication to the supervised injecting facility for clients to collect during their next visit.
Follow‐up HCV RNA testing (to assess treatment response) was scheduled for the end of treatment and for twelve weeks after treatment.
Data collection
De‐identified data were extracted from the supervised injecting facility clinical database, the client management system, and personal electronic medical records: demographic characteristics, markers of social vulnerability (homelessness, contact telephone number, listed next of kin, history of imprisonment, self‐reported psychiatric illness, significant alcohol use [more than four standard drinks on most days]), number of injecting facility visits and substance use details, past hepatitis C treatment, testing for anti‐HCV antibody, HCV RNA, HBV, HIV, ALT, and AST, full blood examination, liver fibrosis assessment, DAA initiation, and treatment outcomes. In 21 cases in which HCV RNA was detected but no anti‐HCV antibody result was recorded, anti‐HCV positivity was assumed. Information about medication dispensing was checked against hospital pharmacy dispensing records.
To evaluate associations between characteristics and client engagement with hepatitis C testing, comparator data were extracted from the supervised injecting facility client management system for people for whom hepatitis C testing was not recorded (demographic characteristics, homelessness, history of imprisonment, self‐reported psychiatric illness, alcohol use, number of injecting facility visits). Data were only included in analyses when complete client data were available.
Study outcomes
The primary outcome was the proportion of people tested for hepatitis C (anti‐HCV antibody). Secondary outcomes were the proportions of people positive for anti‐HCV antibody or HCV RNA, the proportion of eligible people prescribed DAAs, end‐of‐treatment response (undetectable serum HCV RNA at treatment completion), and sustained virological response (undetectable serum HCV RNA twelve weeks or more after treatment conclusion). Re‐infection was defined as detectable HCV RNA after a confirmed sustained virological response or with a change in HCV genotype after treatment. Virological failure was defined as treatment leading to an end‐of‐treatment response but followed by detectable serum HCV RNA (same genotype) after treatment. Sustained virological response was calculated only for people confirmed to have started treatment and for whom complete follow‐up data were available. Incomplete treatment adherence was defined as the client reporting that they had taken less than 90% of the prescribed DAA course or had interrupted treatment for more than four weeks.
Statistical analysis
Continuous data are summarised as medians with interquartile ranges (IQRs), categorical data as frequencies and proportions. The number of injecting facility visits was evaluated both as a continuous and as a categorical variable. The statistical significance of between‐group differences was assessed in Fisher exact and χ2 tests (categorical data) or Mann–Whitney U tests (non‐parametric continuous data). The statistical significance of differences between clients who were or were not tested for HCV was assessed in univariate and multivariate logistic regression analyses (reported as adjusted odds ratios [aORs] with 95% confidence intervals [CIs]). P < 0.05 was deemed statistically significant. Analyses were conducted in Stata 17.0.
Ethics approval
This study was approved by the St Vincent's Hospital, Melbourne, Human Research Ethics Committee (LLR 052/20).
Results
Of 4649 people who attended the supervised injecting facility during 2018–2020, 321 were tested for hepatitis C (7%) (Box 1). Similar numbers were tested during the first (167 people) and second years (154 people) of the program, but the coronavirus disease 2019 (COVID‐19) pandemic disrupted clinical services during the second year; only two people were tested during 19 March – 30 June 2020.
No next of kin was recorded for 137 clients (43%), no fixed address for 127 (40%), and no contact telephone number for 109 (34%); 194 reported a psychiatric illness (60%), 197 a history of imprisonment (61%). An earlier hepatitis C diagnosis was reported by 165 people (51%), and 64 (20%) reported previous hepatitis C treatment (Box 2).
Compared with 1856 clients not tested for hepatitis C, the median age of the 321 people who were tested was slightly higher (45 years; IQR, 39–52 years v 43 years; IQR, 38–50 years), and larger proportions reported no fixed address (127, 40% v 450, 24%), psychiatric illness (194, 60% v 232, 13%), or significant alcohol use (57, 18% v 195, 11%). In multivariate analyses, tested people were more likely than untested clients to report psychiatric illness (aOR, 9.65; 95% CI, 7.26–12.8), have no reported fixed address (aOR, 1.59; 95% CI, 1.18–2.14), and to report significant alcohol use (aOR, 1.57; 95% CI, 1.06–2.32). The median number of injecting facility visits was larger for those tested for hepatitis C (101; IQR, 31–236) than for those who were not (20; IQR, 3–90) (Box 3).
Clients tested for hepatitis C virus
Of the 321 people tested for hepatitis C, 279 were seropositive for anti‐HCV antibody (87%), of whom 143 were also positive for HCV RNA (51%) (Box 4). Eight of 321 people were HBV‐positive (3%), four were HIV‐positive (1%).
Liver fibrosis was assessed in 134 of the 143 HCV RNA‐positive people (94%). APRI scores were available for 129 of the 134; 27 people had APRI scores of 1.0 or more and were referred for transient elastography, of whom eleven underwent the procedure (41%); a further five clients underwent opportunistic on‐site transient elastography as their sole cirrhosis assessment. Cirrhosis was clinically identified in fourteen people with hepatitis C (10%); five attended the hospital for follow‐up hepatology clinic appointments, and three underwent liver ultrasound screening for hepatocellular carcinoma.
Hepatitis C virus therapy and outcomes
DAA therapy was prescribed for 126 of 143 HCV RNA‐positive clients (88%); 103 were triaged to the simple care pathway (82%), 23 (18%) to the complex care pathway (nineteen prescribed DAAs after remote consultations, four with face‐to‐face clinical reviews). Seventeen people (12%) had been lost to follow‐up before DAA therapy could be prescribed. The median time from hepatitis C testing to DAA prescribing was 38 days (IQR, 18–75 days). The most frequently prescribed regimens were sofosbuvir/velpatasvir (85 people, 67%) and glecaprevir/pibrentasvir (23, 18%). Of those prescribed DAAs, dispensing was confirmed for 103 people (82%), non‐initiation of prescribed treatment was confirmed for 13 (10%), and ten were lost to follow‐up (8%).
Complete treatment data were available for 54 people who commenced DAA therapy; sustained virological response was achieved in 41 (ie, hepatitis C cure; 76%). Seventy‐two people (57%) were lost to follow‐up before reaching the sustained virological response time point. The most frequent reason for ineffectiveness of DAA therapy was incomplete treatment adherence (eight people). Four instances of re‐infection and one of virological failure were identified. The proportions of people cured did not differ by hepatitis C genotype (genotype 3 v other) or cirrhosis status (Box 5).
Discussion
We report the successful implementation of a comprehensive, on‐site nurse‐led model of care offering hepatitis C testing, liver fibrosis assessment, and treatment for people who inject drugs at a supervised injecting facility. Such facilities provide a unique harm reduction service and a valuable opportunity to engage a marginalised group of people with hepatitis C testing and treatment. The people in our study attended the supervised injecting facility frequently, and reported high rates of homelessness, imprisonment, mental illness, and heavy alcohol consumption. Although DAA therapy has been available for a number of years, only 20% of people had been treated for hepatitis C. Our model removed a number of barriers that make it difficult for people who inject drugs to engage with standard hepatitis C care pathways15 by providing convenient care in a place that people who inject drugs frequent.
Our findings confirm that the prevalence of hepatitis C among clients of a supervised injecting facility is high; 87% of tested people were anti‐HCV antibody‐positive, 51% of whom were also HCV RNA‐positive. Retention in the cascade of care from diagnosis to DAA prescribing was good: 88% of HCV RNA‐positive people were prescribed DAAs. An important enabler was the nurse‐led outreach model that provides on‐site testing and treatment decision making. Nurse‐led models of care have been successful in other institutions in Australia where the prevalence of hepatitis C is high, including prisons, mental health services, and alcohol and drug services.4,16 The availability of other services at the supervised injecting facility, including harm reduction, housing, opioid agonist therapy, and care coordination, may also have contributed to the success of the program.
The rate of sustained virological response among people confirmed to have started treatment and for whom complete follow‐up data were available was 76%. This was lower than reported by another Australian study (95%),17 but genuine virological failure was rare; treatment non‐adherence and re‐infection were more frequent causes of treatment failure. Overall, our findings confirm the high efficacy of DAA treatment, but also indicate the importance of supporting treatment adherence and harm reduction for people who inject drugs.
Only 7% of people who attended the supervised injecting facility over two years were tested for hepatitis C. This may reflect the time‐limited availability of testing, but more likely the difficulties in engaging marginalised people and the sometimes challenging nature of venepuncture in this group. Clients who were tested visited the facility more frequently than those who were not and consequently had greater opportunity to be tested. An important question is whether HCV RNA point‐of‐care tests might be useful for increasing the testing of people who attend less frequently. The recent Therapeutic Goods Administration approval of point‐of‐care HCV RNA fingerstick tests provides an exciting opportunity to further remove barriers to hepatitis C care, and models incorporating point‐of‐care HCV RNA testing have been highly acceptable in other settings.18,19 Further strategies to increase engagement, including financial incentives and “blitz” days supported by increased resources and a focus on hepatitis C care, should also be considered.20,21 Given the relatively high rates in our study of homelessness, psychiatric illness, and heavy alcohol use among people who were tested for hepatitis C, they may be more likely to use the supervised injecting facility as their primary health care service. Promoting engagement by all clients of such facilities with care should be further investigated.
Limitations
The retrospective design of our study means it is subject to recall bias, and our findings may have been influenced by missing data. Further, the comparisons we describe were not based on randomised groups, limiting the conclusions that can be drawn regarding predictors of hepatitis C testing. The high rate of loss to follow‐up meant that complete follow‐up data were available for only 54 people who commenced DAA therapy. Such loss is typical for services caring for people who inject drugs, but the findings of an earlier Australian study suggest that the key step in the cascade of care for hepatitis C is the prescribing and dispensing of DAA treatment.22
Conclusion
Our study is the first to report the successful integration of a comprehensive hepatitis C model of care, including testing and DAA therapy, into a supervised injecting facility. Such programs, offering testing and treatment to people who inject drugs and consequently at high risk of hepatitis C, will play a crucial role in eliminating the disease in Australia by 2030.
Box 1 – Cascade of hepatitis C virus (HCV) care for 4649 people who attended the Melbourne supervised injecting facility, 30 June 2018 – 30 June 2020
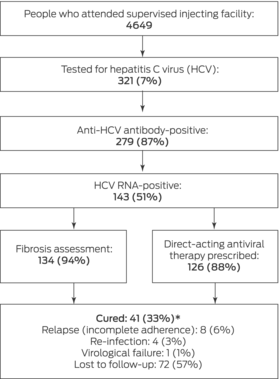
* Cure among people with complete follow‐up data: 41 of 54 (76%).
Box 2 – Baseline characteristics of 321 people who attended the Melbourne supervised injecting facility and were tested for hepatitis C virus (HCV) infection, 30 June 2018 – 30 June 2020
|
Characteristics |
Value |
||||||||||||||
|
|
|||||||||||||||
|
Age (years) median (IQR) |
45 (39–52) |
||||||||||||||
|
Sex (men) |
254 (79%) |
||||||||||||||
|
Aboriginal or Torres Strait Islander people |
40 (12%) |
||||||||||||||
|
Markers of social vulnerability |
|
||||||||||||||
|
No fixed address |
127 (40%) |
||||||||||||||
|
No contact phone listed |
109 (34%) |
||||||||||||||
|
Next of kin listed |
184 (57%) |
||||||||||||||
|
History of imprisonment |
197 (61%) |
||||||||||||||
|
Psychiatric illness (self‐reported) |
194 (60%) |
||||||||||||||
|
History of alcohol misuse |
57 (18%) |
||||||||||||||
|
Opiate substitution therapy |
|
||||||||||||||
|
At time of first visit to the supervised injecting facility |
80 (25%) |
||||||||||||||
|
Ever |
201 (63%) |
||||||||||||||
|
Number of supervised injecting facility visits, median (IQR) |
101 (31–236) |
||||||||||||||
|
Preferred drug injected |
|
||||||||||||||
|
Heroin |
297 (93%) |
||||||||||||||
|
Methamphetamine |
8 (2%) |
||||||||||||||
|
Other |
2 (1%) |
||||||||||||||
|
Not recorded |
14 (4%) |
||||||||||||||
|
Age of first injecting drug use (years), median (IQR) |
18 (15–22) |
||||||||||||||
|
Previous hepatitis C care |
|
||||||||||||||
|
Diagnosis |
165 (51%) |
||||||||||||||
|
Treatment |
64 (20%) |
||||||||||||||
|
Direct‐acting antiviral therapy (with or without ribavirin) |
44/64 (69%) |
||||||||||||||
|
Interferon (with or without ribavirin) |
14/64 (22%) |
||||||||||||||
|
Unknown |
9/64 (14%) |
||||||||||||||
|
Anti‐HCV antibody‐positive |
279/321 (87%) |
||||||||||||||
|
HCV RNA‐positive, by group |
|
||||||||||||||
|
People with previous HCV treatment |
25/64 (39%) |
||||||||||||||
|
People without previous HCV treatment |
118/257 (46%) |
||||||||||||||
|
Anti‐HCV antibody‐positive clients |
143/279 (51%) |
||||||||||||||
|
HBV serology |
|
||||||||||||||
|
HBsAg detected |
8 (2%) |
||||||||||||||
|
Anti‐HBsAg detected |
198 (62%) |
||||||||||||||
|
Anti‐HBcAg detected |
109 (34%) |
||||||||||||||
|
HIV‐positive |
4 (1%) |
||||||||||||||
|
Cirrhosis |
21 (7%) |
||||||||||||||
|
Among HCV RNA‐positive clients |
14/143 (10%) |
||||||||||||||
|
|
|||||||||||||||
|
HBV, hepatitis B virus (HBsAg = surface antigen, HBcAg = core antigen); HIV, human immunodeficiency virus; IQR = interquartile range. |
|||||||||||||||
Box 3 – Univariate and multivariate logistic regression analyses of baseline characteristics, by engagement with hepatitis C virus (HCV) testing
|
Characteristic |
Tested for HCV |
Not tested for HCV |
Odds ratio (95% CI) |
Adjusted odds ratio* (95% CI) |
|||||||||||
|
|
|||||||||||||||
|
Number of people |
321 |
1856 |
|
|
|||||||||||
|
Age (years) median (IQR) |
45 (39–52) |
43 (38–50) |
1.02 (1.01–1.04) |
1.03 (1.02–1.05) |
|||||||||||
|
Sex (men) |
254 (79%) |
1399 (75%) |
0.81 (0.60–1.08) |
– |
|||||||||||
|
Aboriginal or Torres Strait Islander people |
40 (12%) |
255 (14%) |
0.90 (0.63–1.28) |
– |
|||||||||||
|
No fixed address |
127 (40%) |
450 (24%) |
2.01 (1.57–2.57) |
1.59 (1.18–2.14) |
|||||||||||
|
History of imprisonment |
197 (61%) |
1057 (57%) |
1.20 (0.94–1.53) |
– |
|||||||||||
|
Psychiatric illness (self‐reported) |
194 (60%) |
232 (13%) |
10.7 (8.22–13.9) |
9.65 (7.26–12.8) |
|||||||||||
|
History of alcohol misuse |
57 (18%) |
195 (11%) |
1.84 (1.33–2.54) |
1.57 (1.06–2.32) |
|||||||||||
|
Number of supervised injecting facility visits† |
|
|
|
|
|||||||||||
|
1–5 |
29/310 (9%) |
573/1825 (31%) |
1 |
1 |
|||||||||||
|
6–30 |
46/310 (15%) |
445/1825 (24%) |
2.04 (1.23–3.43) |
1.83 (1.09–3.07) |
|||||||||||
|
31–110 |
85/310 (27%) |
418/1825 (23%) |
4.02 (2.55–6.47) |
3.50 (2.18–5.64) |
|||||||||||
|
More than 110 |
150/310 (48%) |
389/1825 (21%) |
7.62 (4.97–12.0) |
6.59 (4.18–10.4) |
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; IQR = interquartile range. * Adjusted for age, no fixed address, psychiatric illness, history of alcohol misuse and number of supervised injecting facility visits. † The number of supervised injecting facility visits was not available for all people. |
|||||||||||||||
Box 4 – Characteristics of the 143 people positive for hepatitis C virus (HCV) RNA
|
Characteristic |
Value |
||||||||||||||
|
|
|||||||||||||||
|
HCV RNA‐positive |
143 |
||||||||||||||
|
HCV genotype |
|
||||||||||||||
|
1a |
61 (43%) |
||||||||||||||
|
1b |
7 (5%) |
||||||||||||||
|
2 |
2 (1%) |
||||||||||||||
|
3 |
67 (47%) |
||||||||||||||
|
6 |
2 (1%) |
||||||||||||||
|
Not available/missing data |
4 (3%) |
||||||||||||||
|
HCV viral load (IU/mL), median (IQR) |
476 000 (129 750–2 117 500) |
||||||||||||||
|
Alanine transaminase* (U/L), median (IQR) |
43 (30–66) |
||||||||||||||
|
Platelet count† (109/L), median (IQR) |
275 (221–340) |
||||||||||||||
|
AST‐to‐platelet ratio index (APRI) ≥ 1.0 |
27/129 (21%) |
||||||||||||||
|
Liver stiffness (transient elastography) |
|
||||||||||||||
|
< 9.5 kPa |
8/16 (50%) |
||||||||||||||
|
9.5–12.5 kPa |
1/16 (6%) |
||||||||||||||
|
> 12.5 kPa |
7/16 (44%) |
||||||||||||||
|
HBV infection (HBsAg‐positive) |
5 (3%) |
||||||||||||||
|
HIV infection |
1 (1%) |
||||||||||||||
|
Cirrhosis‡ |
14 (10%) |
||||||||||||||
|
|
|||||||||||||||
|
AST = aspartate transaminase; HBV = hepatitis B virus (HBsAg = surface antigen); HIV = human immunodeficiency virus; IQR = interquartile range. * Reference interval: 0–35 U/L. † Reference interval: 150–400 x 109/L. ‡ Clients with APRI scores above 1.0 were deemed to be at risk of advanced fibrosis or cirrhosis; those with liver stiffness exceeding 12.5 kPa were deemed to have cirrhosis, as were people who reported a history of cirrhosis or an earlier transient elastography assessment exceeding 12.5 kPa. |
|||||||||||||||
Box 5 – Treatment outcomes for 54 hepatitis C virus (HCV) RNA‐positive people treated with direct‐acting antiviral agents
|
Parameter |
Value |
||||||||||||||
|
|
|||||||||||||||
|
HCV RNA‐negative (sustained virological response) |
41/54 (76%) |
||||||||||||||
|
Genotype |
|
||||||||||||||
|
3 |
20/23 (87%) |
||||||||||||||
|
Other |
21/31 (68%) |
||||||||||||||
|
Cirrhosis |
|
||||||||||||||
|
Yes |
5/5 (100%) |
||||||||||||||
|
No |
36/49 (73%) |
||||||||||||||
|
Virological failure |
1/54 (2%) |
||||||||||||||
|
Re‐infection |
4/54 (7%) |
||||||||||||||
|
Sustained virological response prior to re‐infection |
2/4 (50%) |
||||||||||||||
|
Positive sustained virological response test with genotype switch |
2/4 (50%) |
||||||||||||||
|
HCV RNA‐positive with incomplete treatment adherence |
8/54 (15%) |
||||||||||||||
|
Treatment‐related deaths |
0 |
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 31 October 2021, accepted 27 January 2023
- Michael B MacIsaac1,2
- Bradley Whitton1
- Adrian Hubble1
- Shelley Cogger3
- Matthew Penn3
- Anthony Weeks3
- Kasey Elmore3
- David Pemberton3
- Jenine Anderson3
- Rebecca Howard1
- Una McKeever1
- Timothy Papaluca1,2
- Margaret E Hellard4,5,6
- Mark Stoove4,5
- David Wilson4
- Alisa Pedrana4,5
- Joseph Doyle4,6
- Nico Clark3,7
- Jacinta Holmes1,2
- Alexander J Thompson1,2
- 1 St Vincent's Hospital Melbourne, Melbourne, VIC
- 2 The University of Melbourne, Melbourne, VIC
- 3 North Richmond Community Health, Melbourne, VIC
- 4 The Burnet Institute, Melbourne, VIC
- 5 Monash University, Melbourne, VIC
- 6 Alfred Health, Melbourne, VIC
- 7 The Royal Melbourne Hospital, Melbourne, VIC
Open access
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Alexander Thompson received funding from the National Health and Medical Research Council (NHMRC; Practitioner Fellowship Grant 1142976). This investigation was supported by an NHMRC Program Grant (1132902) and an NHMRC Partnership Grant (1116161). Jacinta Holmes is funded by a University of Melbourne CR Roper Fellowship and an NHMRC program grant.
Margaret Hellard receives funding from Gilead Sciences and Abbvie for investigator‐initiated research. Michael MacIsaac has received speakers’ honoraria from Gilead Sciences. Mark Stoove has received investigator‐initiated research funding from Gilead Sciences and AbbVie and consultant fees from Gilead Sciences for activities unrelated to this work. Joseph Doyle has received investigator‐initiated research funding and speakers’ honoraria from Gilead Sciences and AbbVie. Alisa Pedrana has received investigator‐initiated research funding from Gilead Sciences and AbbVie and speakers’ honoraria from Gilead Sciences. Alexander Thompson has received consulting fees from Gilead, Abbvie, Roche, BMS, Merck, Immunocore, Janssen, Assembly Biosciences, Arbutus, Eisai, Ipsen, and Bayer, speakers’ fees and investigator‐initiated grants from Gilead Sciences.
Received 31 October 2022, accepted 27 January 2023
- 1. World Health Organization. Hepatitis C: key facts. 24 June 2022. https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐c (viewed Oct 2022).
- 2. Giddings HF, Topp L, Middleton M, et al. The epidemiology of hepatitis C in Australia: notifications, treatment uptake and liver transplantations, 1997–2006. J Gastroenterol Hepatol 2009; 24: 1648‐1654.
- 3. Australian Department of Health. Fifth national hepatitis C strategy: 2018–2022. 2018. https://www.health.gov.au/sites/default/files/documents/2022/06/fifth‐national‐hepatitis‐c‐strategy‐2018‐2022.pdf (viewed Oct 2021).
- 4. Harney BL, Brereton R, Whitton B, et al. Hepatitis C treatment in a co‐located mental health and alcohol and drug service using a nurse‐led model of care. J Viral Hepat 2021; 28: 771‐779.
- 5. Sociás ME, Karamouzian M, Parent S, et al. Integrated models of care for people who injectdrugs and live with hepatitis C virus: a systematic review. Int J Drug Policy 2019; 72: 146‐159.
- 6. Alimohammadi A, Holeksa J, Thiam A, et al. Real‐world efficacy of direct‐acting antiviral therapy for HCV Infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis 2018; 5: 120‐127.
- 7. Ford MM, Johnson N, Desai P, et al. From care to cure: demonstrating a model of clinical patient navigation for hepatitis C care and treatment in high‐need patients. Clin Infect Dis 2017; 64: 685‐691.
- 8. Potier C, Laprévote V, Dubois‐Arber F, et al. Supervised injection services: what has been demonstrated? A systematic literature review. Drug Alcohol Depend 2014; 145: 48‐68.
- 9. Semaan S, Fleming P, Worrell C, et al. Potential role of safer injection facilities in reducing HIV and hepatitis C infections and overdose mortality in the United States. Drug Alcohol Depend 2011; 118: 100‐110.
- 10. Wood E. Do supervised injecting facilities attract higher‐risk injection drug users? Am J Prev Med 2005; 29: 126‐130.
- 11. Wood E, Kerr T, Stoltz J, et al. Prevalence and correlates of hepatitis C infection among users of North America's first medically supervised safer injection facility. Public Health 2005; 119: 1111‐1116.
- 12. Van Den Boom W, Del Mar Quiroga M, Fetene DM, et al. The Melbourne safe injecting room attracted people most in need of Its service. Am J Prev Med 2021; 61: 217‐224.
- 13. Medically Supervised Injecting Room Review Panel. Review of the medically supervised injecting room. June 2020. https://content.health.vic.gov.au/sites/default/files/migrated/files/collections/research‐and‐reports/r/review‐of‐the‐medically‐supervised‐injecting‐room‐june‐2020.pdf (viewed Oct 2021).
- 14. Wai CT, Greenson JK, Fontana RJ, et al. A simple non‐invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518‐526.
- 15. Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013; 207 (Suppl 1): S19‐S25.
- 16. Papaluca T, McDonald L, Craigie A, et al. Outcomes of treatment for hepatitis C in prisoners using a nurse‐led, statewide model of care. J Hepatol 2019; 70: 839‐846.
- 17. Yee J, Carson JM, Hajarizadeh B, et al; REACH‐C Study Group. High effectiveness of broad access direct‐acting antiviral therapy for hepatitis C in an Australian real‐world cohort: the REACH‐C study. Hepatol Commun 2022; 6: 496‐512.
- 18. Conway A, Read P, Gilliver R, et al. Awareness of HCV status and preferences for testing and treatment among people with recent injecting drug use at a peer‐led needle and syringe program: the TEMPO pilot study. Viruses 2022; 14: 2463.
- 19. O'Loan J, Young M, Mooney M, et al. Same day delivery! HCV point of care testing in South East Queensland marginalised communities simplifies diagnosis and ensures rapid access to treatment. International Conference on Hepatitis Care in Substance Users conference [virtual], 13–15 October 2021. https://www.inhsu.org/resource/same‐day‐delivery‐hcv‐point‐of‐care‐testing‐in‐southeast‐queensland‐marginalised‐communities‐simplifies‐diagnosis‐and‐ensures‐rapid‐access‐to‐treatment (viewed Feb 2023).
- 20. Chan K, Elsum I, Gold J, et al; Eliminate Hepatitis C Partnership. Increasing hepatitis C testing and linkage to care: results of a testing campaign with incentives at primary care clinics in Melbourne, Australia. J Viral Hepat 2021; 28: 569‐572.
- 21. Palmer AY, Chan K, Gold J, et al; EC Partnership. A modelling analysis of financial incentives for hepatitis C testing and treatment uptake delivered through a community‐based testing campaign. J Viral Hepat 2021; 28: 1624‐1634.
- 22. Iversen J, Dore GJ, Starr M, et al. Estimating the consensus hepatitis C cascade of care among people who inject drugs in Australia: pre and post availability of direct acting antiviral therapy. Int J Drug Policy 2020; 83: 102837.






Abstract
Objective: To evaluate the feasibility of testing and treating people who inject drugs at a supervised injecting facility for hepatitis C virus (HCV) infection.
Design: Retrospective cohort study.
Setting, participants: People who inject drugs who attended the Melbourne supervised injecting facility, 30 June 2018 – 30 June 2020.
Main outcome measures: Proportion of people tested for hepatitis C; proportions of people positive for anti‐HCV antibody and HCV RNA, and of eligible people prescribed direct‐acting antiviral (DAA) treatment; sustained virological response twelve weeks or more after treatment completion.
Results: Of 4649 people who attended the supervised injecting facility during 2018–20, 321 were tested for hepatitis C (7%); 279 were anti‐HCV antibody‐positive (87%), of whom 143 (51%) were also HCV RNA‐positive. Sixty‐four of 321 had previously been treated for hepatitis C (20%), 21 had clinically identified cirrhosis (7%), eight had hepatitis B infections (2%), and four had human immunodeficiency virus infections (1%). In multivariate analyses, people tested for hepatitis C were more likely than untested clients to report psychiatric illness (adjusted odds ratio [aOR], 9.65; 95% confidence interval [CI], 7.26–12.8), not have a fixed address (aOR, 1.59; 95% CI, 1.18–2.14), and to report significant alcohol use (aOR, 1.57; 95% CI, 1.06–2.32). The median number of injecting facility visits was larger for those tested for hepatitis C (101; interquartile range [IQR], 31–236) than for those not tested (20; IQR, 3–90). DAA treatment was prescribed for 126 of 143 HCV RNA‐positive clients (88%); 41 of 54 with complete follow‐up data were cured (76%).
Conclusions: People who attend supervised injecting facilities can be tested and treated for hepatitis C on site. Models that provide streamlined, convenient hepatitis C care promote engagement with treatment in a group in which the prevalence of hepatitis C is high.