Clinical record
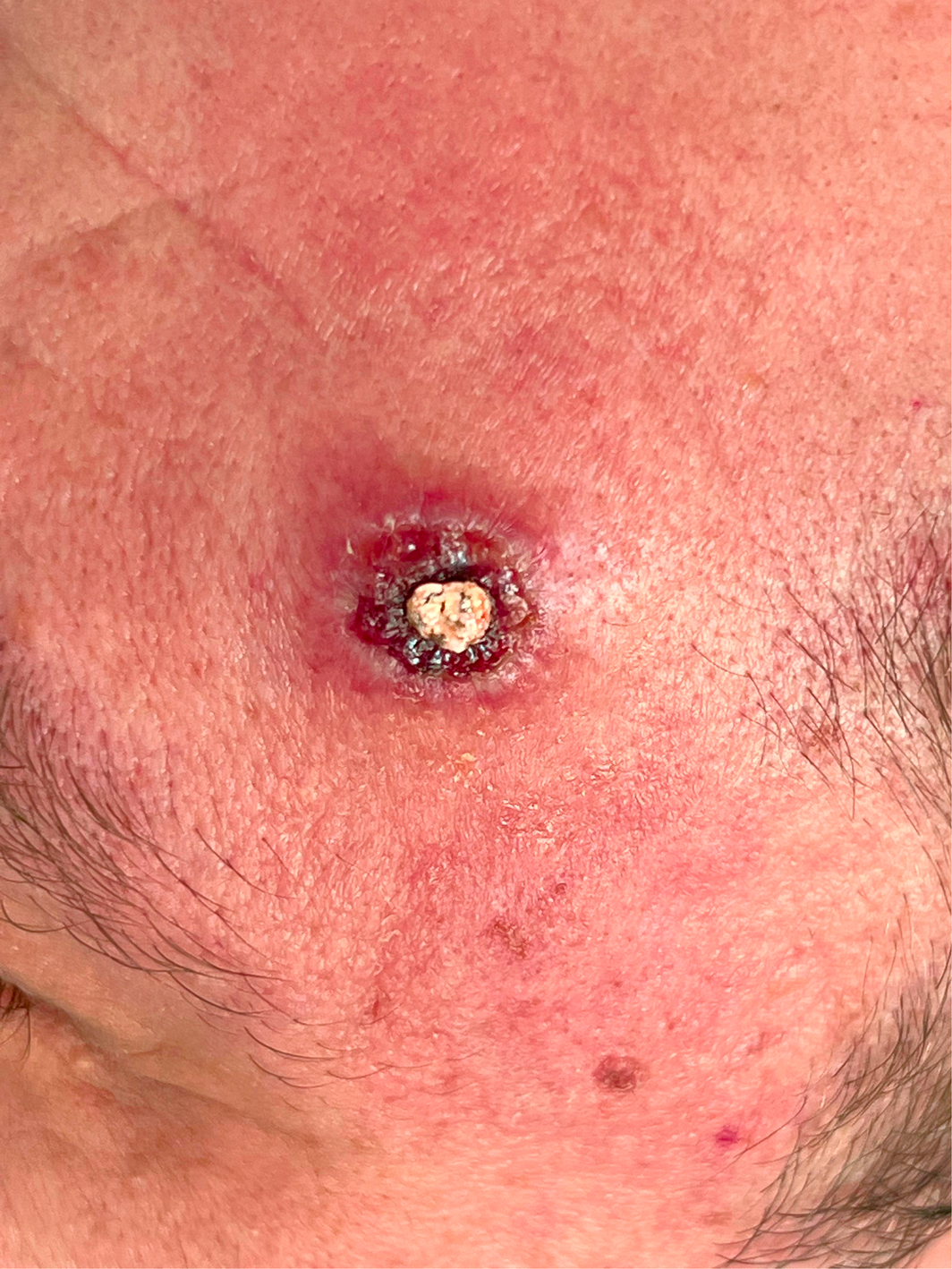
A man in his thirties presented immediately on return from a one‐month trip to Europe with widespread pustular lesions, tender lymphadenopathy, fever, and headache. Initial contact was with his general practitioner, who notified the local Public Health Unit and the Infectious Diseases Unit. He identified as a man who has sex with men. He had a background of human immunodeficiency virus (HIV) infection, which was well controlled (CD4+ cells, 1190 cells/mm3 [reference interval (RI), 560–1580 cells/mm3]; HIV viral load not detected [limit of quantitation, 20 RNA copies/mL]) with bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg daily. He first noticed a lesion resembling a pimple on the forehead while still overseas, which progressed over the six days before his return (Box 1) followed by a clustering of similar lesions over his right buttock, but no mucosal lesions. More lesions then developed over his thigh and hand. He reported intermittent rectal paraesthesia and spasm, in addition to fever and mild generalised headache. Tender inguinal and cervical lymphadenopathy became established, along with fatigue and malaise. He did not report sore throat, cough, diarrhoea, dysuria, or neck stiffness. He had had sexual contact with known cases of mpox (formerly monkeypox) in Europe. He was admitted to hospital in a single negative pressure room, with contact and airborne precautions. Full blood count and chemistry were normal, and C‐reactive protein was 17 mg/L (RI, < 5 mg/L). Testing for Chlamydia trachomatis and Neisseria gonorrhoeae was negative. Swabs were taken from the perianal lesion and sent for National Association of Testing Authorities (NATA)‐accredited in‐house Orthopoxvirus group real‐time polymerase chain reaction (PCR) test targeting the OPG105 gene. Positive by real‐time PCR, monkeypox virus (MPXV) DNA from both the perianal lesion and throat specimens was subsequently confirmed using two additional conventional PCR tests targeting the OPG105 and OPG185 genes. Whole genome sequencing and phylogenetic analyses (Supporting Information) of a genome sequence obtained from the perianal specimen (MPXV_QLD_MX00001_2022, GenBank accession number OP235282) demonstrated placement within the human MPXV (hMPXV) sublineage B.1 of clade IIb and was most closely related to other recent 2022 MPXV sequences from the United States, Europe, Australia and Canada (Box 2). Additional laboratory methods are included in the Supporting Information.
No antiviral therapy was initiated, and his systemic symptoms improved rapidly over 24 hours, at which time he was discharged home to self‐isolate under the care of the coronavirus disease 2019 (COVID‐19) virtual ward. He lived alone and was instructed to stay at home except for essential activities, such as buying groceries or solo outdoor exercise, and to avoid high risk settings and physical and intimate contact with others until all skin lesions had scabbed over.
Discussion
Mpox is caused by MPXV, a double‐stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.1 The incubation period is five to 21 days, and most clinical infections are self‐limiting. Symptoms include rash, fever, lethargy and lymphadenopathy, usually lasting two to four weeks.1 By 5 January 2023, in the current global mpox outbreak, 110 countries have reported cases, including 144 laboratory‐confirmed cases in Australia.2 An international case series reported that 95% of patients presented with a rash (almost two‐thirds having fewer than ten lesions), 62% reported fever, and 56% lymphadenopathy.3 Ninety‐eight per cent of infected individuals were found to be gay or bisexual men, 41% had HIV infection, and MPXV was detected in 91% (29/32) of seminal fluid specimens tested. Concomitant sexually transmissible infections were reported in 29% of cases.
Although MPXV was thought to be mainly transmitted via respiratory droplets and close contact with skin lesions, the current global outbreak is notable for spreading primarily during sexual contact. Fomites (eg, towels, sex toys) may be also involved in MPXV transmission. Public health interventions including case and close contact management should be provided as per the mpox guidelines from the Communicable Diseases Network Australia (CDNA).4 We were able to repurpose the existing virtual ward and public health capacity developed originally for the COVID‐19 pandemic response, demonstrating the legacy of a stronger public health system able to deal with emerging infectious diseases outbreaks.
There are no antiviral therapies for mpox approved by the Therapeutic Goods Administration (TGA). However, the antiviral agent tecovirimat, which inhibits the MPXV envelope wrapping protein, is held in the National Medication Stockpile for the treatment of severe disease and for patients at risk of complications, such as severely immunosuppressed individuals and children less than eight years old.5 Vaccinia immune globulin may be an alternative therapy in pregnancy. Management is generally supportive, with careful attention to analgesia for anogenital and oropharyngeal lesions.
The replication‐incompetent live virus vaccine modified vaccinia Ankara–Bavarian Nordic (MVA‐BN, Jynneos [Bavarian Nordic]) prevents mpox infection, although occasional breakthrough cases are described. In these cases, it may reduce the severity of mpox.6 The vaccine is not registered for use in Australia but is available via a special emergency pathway.
Genetic classification of MPXV was recently revised, with new naming conventions comprising of clade I (formerly Central African) and clade II (formerly West African, further divided into IIa and IIb).7 The current 2022 human outbreak is caused by the hMPXV sublineage B.1 of clade IIb, which might cause less severe disease.
The differential diagnosis for mpox is broad and includes herpes simplex virus infections, chickenpox/shingles, bacterial skin infections, enterovirus infection, syphilis, and medication‐associated rashes. Laboratory diagnosis includes direct MPXV detection by PCR in lesion material (lesion fluid, lesion tissue or skin biopsy). Although not currently recommended, in this case a positive result was also returned from a PCR test performed on a throat swab. There is no TGA‐registered serology test currently available.
Lessons from practice
- The current mpox outbreak was declared a public health emergency of international concern by the World Health Organization.
- Most cases have occurred in men who have sex with men, and 40–50% of cases have occurred in people who are living with human immunodeficiency virus (HIV) infection.
- The clinical presentation is of maculopapular then pustular lesions less than 5 mm wide. Although lesions may occur anywhere and may be low in number, anogenital lesions are a prominent feature of the current outbreak.
- The diagnosis is confirmed by collecting lesion material specimens for Orthopoxvirus polymerase chain reaction testing.
- Most cases can be managed in the community with self‐isolation and adherence to the Communicable Diseases Network Australia National guidelines for Public Health Units.
- Whole genome sequencing and phylogenetic analysis show the current outbreak is caused by monkeypox virus (MPXV) grouping in clade IIb, human MPXV sublineage B.1.
Box 2 – A midpoint‐rooted maximum likelihood tree inferred using the Jukes–Cantor nucleotide substitution model and IQ‐TREE (v1.6.12) from 115 full‐length mpox virus (MPXV) sequences following multiple sequence alignment based on fast Fourier transform (MAFFT, v7.453)*
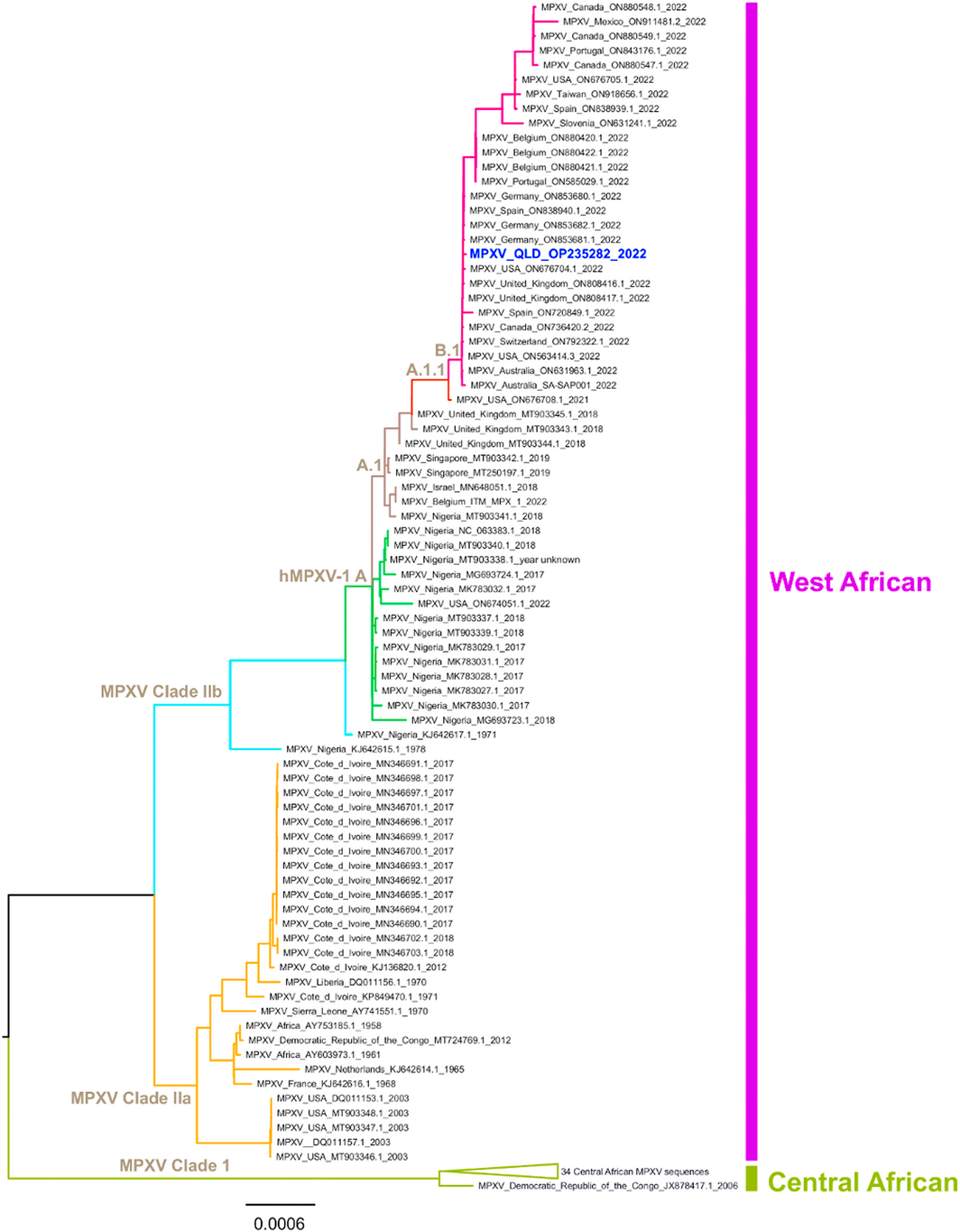
* Alignments were trimmed to remove the 5’ inverted terminal repeat (ITR) sequence and mask several repetitive regions. The newly classified MPXV phylogenetic clades I (formally Central African), IIa and IIb (formally West African) are highlighted, and the human MPXV (h) sublineages A, A.1, A.1.1 and B.1 are named at key nodes and are correspondingly denoted with coloured branches. Clade I includes a collapsed grouping of 34 sequences. The recent Queensland 2022 MPXV sequence (MPXV_QLD_OP235282_2022) is highlighted in bold blue font.
Provenance: Not commissioned; externally peer reviewed.
- 1. World Health Organization. Monkeypox fact sheet. https://www.who.int/news‐room/fact‐sheets/detail/monkeypox (viewed July 2022).
- 2. World Health Organization. Multi‐country outbreak of monkeypox — External Situation Report 13 [5 January 2023]. https://www.who.int/publications/m/item/multi‐country‐outbreak‐of‐mpox‐‐external‐situation‐report‐‐13‐‐‐5‐january‐2023 (viewed 17 Jan 2023).
- 3. Thornhill JP, Barkati S, Walmsley MD, et al. Monkeypox virus infection in humans across 16 countries — April–June 2022. N Engl J Med 2022; 387: 679‐691.
- 4. Australian Government, Department of Health and Aged Care. Monkeypox virus infection — CDNA interim national guidelines for Public Health Units [8 Sept 2022]. https://www.health.gov.au/resources/publications/monkeypox‐virus‐infection‐cdna‐national‐guidelines‐for‐public‐health‐units?language=en (viewed Nov 2022
- 5. Australian Government. Australian human monkeypox treatment guidelines [24 June 2022]. https://www.health.gov.au/sites/default/files/documents/2022/06/monkeypox‐treatment‐guidelines.pdf (viewed Aug 2022).
- 6. Hazra A, Rusie L, Hedburg T, Schneider JA. Human monkeypox virus infection in the immediate period after receiving modified vaccinia Ankara vaccine. JAMA 2022; 328: 2064‐2067.
- 7. Guarner J, Del Rio C, Malani PN. Monkeypox in 2022 — what clinicians need to know. JAMA 2022; 328: 139‐140.






Open access
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Krispin Hajkowicz has received speaking fees, honoraria, advisory board fees, and a research grant from Gilead Sciences, and support from Moderna to attend an educational meeting. Adam Stewart has received speaking fees and honoraria from Gilead Sciences, and support from Pfizer for travel and meeting expenses.