In the first two weeks after their release from prison, people are at greater risk than usual of fatal opioid overdose.1 They are also at greater risk of non‐fatal opioid overdose,2 which is associated with considerable morbidity.3,4 An analysis of linked Queensland ambulance data (non‐fatal opioid overdose‐related ambulance contacts defined as those reporting improved Glasgow Coma Scale [GCS] scores or case notes describing clinical improvement after naloxone administration) estimated that the crude incidence rate of non‐fatal opioid overdose among people released from prison was 4.5 (95% confidence interval [CI], 2.8–7.2) cases per 1000 person‐years.5 However, the study included people who reported never having injected drugs, and thereby probably underestimated non‐fatal opioid overdose incidence among those at greatest risk: people who had injected drugs prior to their imprisonment.
We linked data for a prospective cohort of men who injected drugs at least once a month prior to imprisonment in Victoria6 with ambulance records to estimate the incidence of post‐release non‐fatal opioid overdose. We defined relevant ambulance contacts as those reporting improved GCS scores after naloxone administration. Participants were followed from the recruitment prison release (1 September 2014 – 31 December 2016) until death (National Death Index data linkage) or 31 December 2018. The Alfred Hospital Human Research Ethics Committee (79/12) approved the study protocol.
The mean age of the 400 participants at pre‐release baseline interview was 36 years (standard deviation, 8 years; range, 19–64 years), 355 reported having injected heroin at least once (86%), including 220 (55%) in the month preceding imprisonment, and 121 reported current opioid agonist therapy (OAT; 30%).6 Twenty‐seven people died during the observation period. Forty‐seven participants (12%) reported a total of 70 non‐fatal opioid overdose events (range, 0–4 events per person) during 1222 person‐years of observation (incidence rate, 57 [95% CI, 45–72] per 1000 person‐years). Non‐fatal opioid overdose incidence was highest during the 14 days following release (nine events; incidence rate, 587 [95% CI, 306–1129] per 1000 person‐years) (Box).
The incidence of post‐release non‐fatal opioid overdose in our study was almost 13 times as high as that in the recent Queensland study,5 but we probably underestimated it by including only events attended by ambulances. Our findings indicate that better harm reduction measures are needed during and after release from prison to reduce the overdose rate. Retention in OAT programs substantially reduces fatal overdose risk,1 and has similar benefits for averting non‐fatal opioid overdose.2 Prior to release, people receiving OAT in Victorian prisons are referred to private primary health care providers for treatment, as there are no public clinics. The number of people in Victorian prisons receiving OAT increased from 842 in 2020 to 1230 in 2021 (46% increase),7 while the proportion of community‐based general practitioners prescribing OAT fell from 23% to 12%;7,8,9 66% of Victorian prescribers (698 of 1059) were treating five or fewer patients each in 2021.7
Re‐introducing public OAT clinics in Victoria could improve accessibility for some, and meet the complex needs of people recently released from prison. However, as equitable access will remain a problem because of geographic and capacity constraints, OAT prescribing should become a routine part of general practice.10 Prison‐based take‐home naloxone programs also reduce post‐release opioid‐related mortality.11 A take‐home naloxone pilot program was initiated in some Victorian prisons in 2020; our findings suggest that it should be maintained. Finally, as dispensing fees and other costs (eg, travel) reduce retention in OAT programs,12 the affordability of OAT must be improved.
Box – Crude incidence of non‐fatal opioid overdose after release from Victorian prisons (1 September 2014 – 31 December 2016*) of men who had injected drugs at least once a month prior to their imprisonment
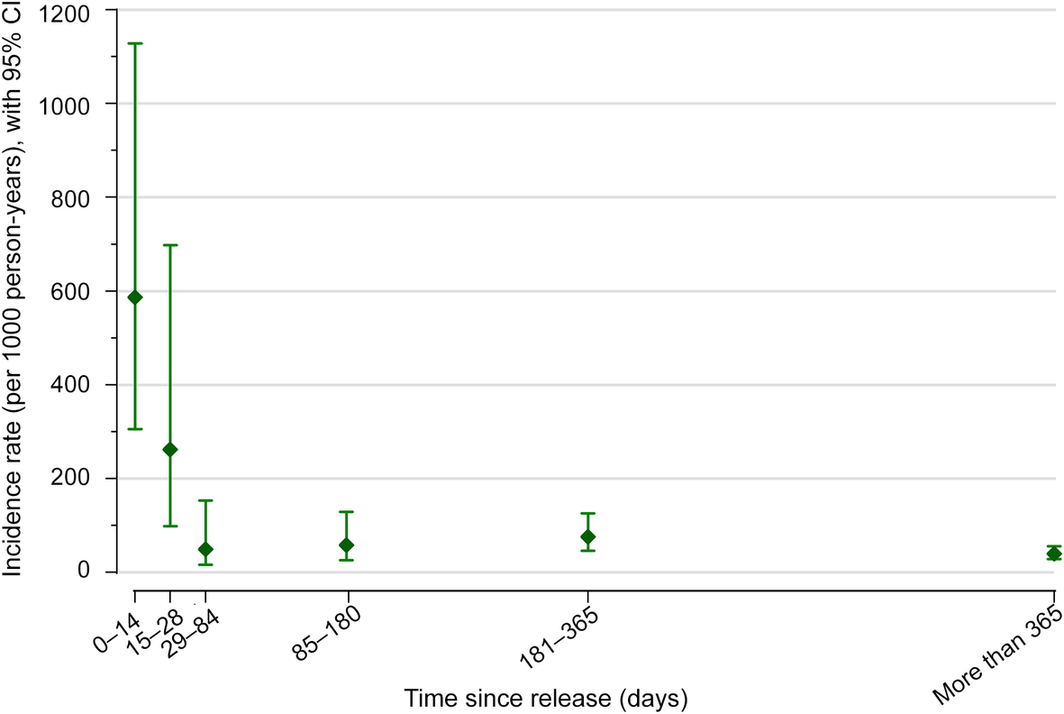
CI = confidence interval.
Number of events by period: 0–14 days, nine; 15–28 days, four; 29–84 days, three; 85–180 days, six; 181–365 days, 15; more than 365 days, 33.
* All baseline interviews (recruitment) were undertaken by 30 June 2016, but release was subsequently delayed for a small number of participants; the final participants were released in December 2016.
Received 23 June 2022, accepted 23 September 2022
- 1. Degenhardt L, Larney S, Kimber J, et al. The impact of opioid substitution therapy on mortality post‐release from prison: retrospective data linkage study. Addiction 2014; 109: 1306‐1317.
- 2. Keen C, Kinner SA, Young JT, et al. Periods of altered risk for non‐fatal drug overdose: a self‐controlled case series. Lancet Public Health 2021; 6: e249‐e259.
- 3. Warner‐Smith M, Darke S, Day C. Morbidity associated with non‐fatal heroin overdose. Addiction 2002; 97: 963‐967.
- 4. Jones NR, Hickman M, Larney S, et al. Hospitalisations for non‐fatal overdose among people with a history of opioid dependence in New South Wales, Australia, 2001–2018: findings from the OATS retrospective cohort study. Drug Alcohol Depend 2021; 218: 108354.
- 5. Keen C, Young JT, Borschmann R, Kinner SA. Non‐fatal drug overdose after release from prison: a prospective data linkage study. Drug Alcohol Depend 2020; 206: e107707. [Cited here: supplementary table S4].
- 6. Kirwan A, Curtis M, Dietze P, et al. The Prison and Transition Health (PATH) Cohort Study: study protocol and baseline characteristics of a cohort of men with a history of injecting drug use leaving prison in Australia. J Urban Health 2019; 96: 400‐410.
- 7. Australian Institute of Health and Welfare. National Opioid Pharmacotherapy Statistics Annual Data Collection. Updated 30 Mar 2022. https://www.aihw.gov.au/reports/alcohol‐other‐drug‐treatment‐services/national‐opioid‐pharmacotherapy‐statistics/contents/prescribers (viewed June 2022).
- 8. Medical Board of Australia. Registration data table: June 2021. 10 Aug 2021. https://www.medicalboard.gov.au/news/statistics.aspx (viewed June 2022).
- 9. Medical Board of Australia. Registration data table: June 2020. 27 Aug 2020. https://www.medicalboard.gov.au/news/statistics.aspx (viewed June 2022).
- 10. Prathivadi P, Sturgiss EA. When will opioid agonist therapy become a normal part of comprehensive health care? Med J Aust 2021; 214: 504‐505.e1. https://www.mja.com.au/journal/2021/214/11/when‐will‐opioid‐agonist‐therapy‐become‐normal‐part‐comprehensive‐health‐care
- 11. Bird SM, McAuley A, Perry S, Hunter C. Effectiveness of Scotland's National Naloxone Programme for reducing opioid‐related deaths: a before (2006–10) versus after (2011–13) comparison. Addiction 2016; 111: 883‐891.
- 12. Zahra E, Chen R, Nielsen S, et al. Examining the cost and impact of dosing fees among clients in opioid agonist treatment: results from a cross‐sectional survey of Australian treatment clients. Drug Alcohol Rev 2022; 41: 841‐850.






Open access
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
The Prison and Transition Health Cohort Study was funded by a National Health and Medical Research Council (NHMRC) project grant (APP1029915). We acknowledge the participants in the study for the time and knowledge they contributed. We thank the Burnet Institute fieldwork team for their tireless efforts with data collection. We also acknowledge Ambulance Victoria, the Victorian Department of Justice and Community Safety, and the Australian Institute of Health and Welfare for their support.
Michael Curtis holds an NHMRC postgraduate award and a Monash Addiction Research Centre PhD top‐up scholarship. Mark Stoove and Paul Dietze hold NHMRC Senior Research Fellowships. We gratefully acknowledge the support provided to the Burnet Institute by the Victorian Government Operational Infrastructure Support Program.
Mark Stoove has received investigator‐initiated funding from Gilead Sciences, AbbVie, and Bristol Myers Squibb, and consultant fees from Gilead Sciences for activities unrelated to this work. Paul Dietze has received investigator‐driven funding from Gilead Sciences for work related to hepatitis C treatment and an untied educational grant from Indivior for work related to the introduction of buprenorphine/naloxone in Australia. He has also served as an unpaid member of an advisory board for an intranasal naloxone product.