Myocarditis in adolescents and young adults following the administration of coronavirus disease 2019 (COVID‐19) mRNA vaccines has been reported.1 Vaccination of 12–16‐year‐old adolescents with Comirnaty (tozinameran, Pfizer–BioNTech) and Spikevax (elasomeran, Moderna) was approved in Australia on 22 July 2021 and 3 September 2021 respectively.2 In this report, we describe the initial diagnosis, imaging findings, and short term outcomes for adolescents who presented with COVID‐19 vaccine‐associated myocarditis to the Monash Children’s Hospital, a tertiary centre in Melbourne with a paediatric cardiology service. The Monash Health human research ethics committee (QA/81618/MonH‐2021‐291293) approved the study.
We included adolescents (12–18 years old) who presented with typical symptoms of myocarditis associated with troponin rise (> 15 ng/L) within 28 days of first or second doses of COVID‐19 mRNA vaccines during 1 August – 31 December 2021. Myocarditis was defined by standard criteria.3 All patients underwent electrocardiography (ECG), echocardiography, and cardiac magnetic resonance (CMR) imaging. CMR images (T2 and late gadolinium enhancement imaging sequences) were acquired with a 1.5 T scanner (Avanto‐Fit, Siemens Healthineers; 32 patients) or a 3 T scanner (Ingenia, Philips Healthcare; one patient). All patients were followed up (as outpatients) two to three weeks after their initial presentation.
None of the 33 included patients presented with congestive heart failure or required intensive care treatment, inotropic support, or immunoglobulin or steroid therapy (Box 1). Fourteen patients (42%) had rising troponin levels at presentation; eight had ECG changes typical for pericarditis, but no arrhythmias were detected by inpatient telemetry. Left ventricular systolic function was normal at presentation in 29 patients and mildly impaired in four (Box 2), and was normal in all patients by follow‐up. Tissue Doppler velocity, a marker of cardiac function, was normal for the thirty patients with technically satisfactory data; the median global longitudinal strain value was normal (20%; interquartile range [IQR], 18.5–20%).
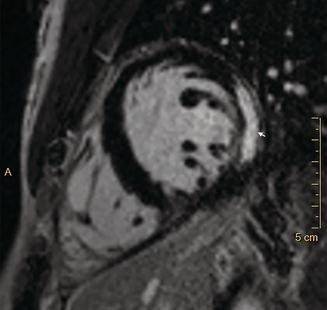
CMR imaging was performed early in most admissions (median, 2 days; IQR, 1–2.5 days); the median interval between peak troponin level and CMR was one day (IQR, 1–2 days). CMR findings were abnormal in 27 of 32 patients (contrast medium could not be administered to one patient because of anxiety), showing late gadolinium enhancement in a patchy subepicardial to transmural pattern, and was especially marked in the inferolateral left ventricular free wall (Box 3). Evidence of oedema in corresponding segments was detected by T2‐weighted CMR in 22 of 32 patients, meeting the Lake Louise criteria for myocarditis.4 The right ventricular apex was affected in isolation in one patient. A small or trace pericardial effusion was noted in 25 of 33 patients, and pericardial enhancement in two of 33 patients. Myocarditis (CDC criteria1) was confirmed for 22 of 32 patients and was probable for ten of 32 patients.
Thirty‐two patients improved in hospital after treatment with high dose ibuprofen (10 mg/kg/dose; typical, 6–8 × 600 mg/dose [maximum, 2400 mg/day]) for one week or until symptom resolution and proton pump inhibitor therapy (omeprazole, 20 mg daily). The median hospital stay was 2.3 days (IQR, 1.9–3.0 days). Two weeks’ bed rest was recommended to all patients, followed by a gradual return to normal activities over three months.
Our vaccine‐associated myocarditis study is the largest reported for a single children’s hospital. Only one of our participants had a history of prior SARS‐CoV‐2 infection, so background immunity is unlikely to have influenced the adverse event profile of vaccination. COVID‐19 mRNA vaccine‐associated myocarditis has a mild, self‐resolving clinical course, in contrast to reported complications and long term sequelae associated with COVID‐19, such as multisystem inflammatory syndrome in children, and other forms of myocarditis.5,6 The long term consequences of myocardial injury with vaccine‐associated myocarditis nevertheless warrant further investigation.
Box 1 – Presentation characteristics of 33 adolescents with COVID‐19 vaccine‐associated myocarditis, Melbourne, 1 August – 31 December 2021
|
Characteristic |
Number/value |
||||||||||||||
|
|
|||||||||||||||
|
Patients |
33 |
||||||||||||||
|
Sex |
|
||||||||||||||
|
Boys |
27 (82%) |
||||||||||||||
|
Girls |
6 (18%) |
||||||||||||||
|
Age (years), median (IQR) |
14.6 (13.0–16.4) |
||||||||||||||
|
COVID‐19 vaccine |
|
||||||||||||||
|
Pfizer–BioNTech |
28 (85%) |
||||||||||||||
|
Moderna |
5 (15%) |
||||||||||||||
|
Vaccine dose |
|
||||||||||||||
|
First |
6 (18%) |
||||||||||||||
|
Second |
27 (82%) |
||||||||||||||
|
Vaccination to presentation (days) |
|
||||||||||||||
|
Median (IQR) |
3 (3–4.5) |
||||||||||||||
|
Range |
2–26 |
||||||||||||||
|
Symptoms |
|
||||||||||||||
|
Chest pain |
33 (100%) |
||||||||||||||
|
Fever |
4 (12%) |
||||||||||||||
|
Shortness of breath |
2 (6%) |
||||||||||||||
|
Headache |
7 (21%) |
||||||||||||||
|
Myalgia |
6 (18%) |
||||||||||||||
|
Palpitations |
3 (9%) |
||||||||||||||
|
Other symptom |
6 (18%) |
||||||||||||||
|
Length of hospital stay (days), median (IQR) |
2.3 (1.9–3.0) |
||||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019; IQR = interquartile range. |
|||||||||||||||
Box 2 – Clinical characteristics of 33 adolescents with COVID‐19 vaccine‐associated myocarditis, Melbourne, 1 August – 31 December 2021
|
Characteristic |
Number/value |
||||||||||||||
|
|
|||||||||||||||
|
Biochemistry |
|
||||||||||||||
|
Troponin, peak level (ng/L), median (IQR) |
2837 (1181–7836) |
||||||||||||||
|
C‐reactive protein* (mg/L), median (IQR) |
16 (6.5–44.5) |
||||||||||||||
|
Electrocardiography |
|
||||||||||||||
|
Normal |
25 (76%) |
||||||||||||||
|
Significant ST elevation |
6 (18%) |
||||||||||||||
|
T wave changes |
2 (6%) |
||||||||||||||
|
Left ventricular ejection fraction |
|
||||||||||||||
|
Normal (≥ 55%) |
29 (88%) |
||||||||||||||
|
Mild dysfunction (45–54%) |
4 (12%) |
||||||||||||||
|
Cardiac magnetic resonance imaging (CMR) (diagnostic) |
|
||||||||||||||
|
Admission to CMR (days), median (IQR) |
2 (1–2.5) |
||||||||||||||
|
Myocardial oedema |
24 (73%) |
||||||||||||||
|
Late gadolinium enhancement† |
27 (84%) |
||||||||||||||
|
Pericardial effusion (small/trace) |
25 (76%) |
||||||||||||||
|
Pericardial enhancement |
2 (6%) |
||||||||||||||
|
Lake Louise criteria for myocarditis4 |
22 (69%) |
||||||||||||||
|
CDC case definition of myocarditis1,† |
|
||||||||||||||
|
Confirmed |
22 (69%) |
||||||||||||||
|
Probable |
10 (31%) |
||||||||||||||
|
|
|||||||||||||||
|
CDC = Centers for Disease Control and Prevention (United States); IQR = interquartile range. * Reference interval: < 5 mg/L; data available for thirty patients only, for 25 of whom the value exceeded the reference interval. † Data available for thirty‐two patients only. |
|||||||||||||||
Received 4 January 2022, accepted 20 April 2022
- 1. Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID‐19 vaccination in adolescents and young adults. Circulation 2022; 45: 345‐356.
- 2. Therapeutic Goods Administration (Australian Department of Health). COVID‐19 vaccine: provisional registrations. 20 June 2022. https://www.tga.gov.au/node/936162 (viewed July 2022).
- 3. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID‐19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices, United States, June 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 977‐982.
- 4. Ferreira VM, Schulz‐Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018; 72: 3158‐3176.
- 5. Feldstein LR, Tenforde MW, Friedman KG, et al; Overcoming COVID‐19 Investigators. Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS‐C) compared with severe acute COVID‐19. JAMA 2021; 325: 1074‐1087.
- 6. Klugman D, Berger JT, Sable CA, et al. Pediatric patients hospitalized with myocarditis: a multi‐institutional analysis. Pediatr Cardiol 2010; 31: 222‐228.






Open access
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
Elys Green and Parvez Patel (MonashHeart) assisted with data collection. We also thank John Troupis and Andrew Kemp (Monash Imaging) for timely CMR imaging access.
No relevant disclosures.