During 2020, 28 327 severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections were notified in Australia, of which 3838 (13.5%) were in people aged 0–19 years (data supplied by National Interoperable Notifiable Diseases Surveillance System, NINDSS) (Box 1). Most SARS‐CoV‐2 infections in children are asymptomatic or mild, and infection rates are probably higher than indicated by notifications that rely on presentations for testing. Knowledge of age‐specific seroprevalence throughout the pandemic provides insights into viral transmission and can guide policies on vaccination and school closures. Healthy children are frequently underrepresented in seroprevalence studies because they present for health care infrequently and investigators are reluctant to perform painful procedures such as venepuncture. Residual blood specimens from samples collected for other purposes are often inadequate for testing; for a Sydney SARS‐CoV‐2 serosurvey based on residual blood (in early 2020), the only age group for which the required sample size was not achieved was children aged 0–9 years.1
We assessed the seroprevalence of SARS‐CoV‐2‐specific antibodies in children aged 0–19 years in blood opportunistically collected during anaesthesia from patients undergoing elective surgery at seven hospitals in the Paediatric Active Enhanced Disease Surveillance network (PAEDS; www.paeds.org.au) during 3 November 2020 – 12 March 2021 (Box 2), a period of very low SARS‐CoV‐2 infection notifications (Box 1). Parents or guardians (for people under 18 years of age) or participants (aged 18 or 19 years) provided written consent; assent was also obtained from adolescents aged 12 years or more. The Sydney Children’s Hospital Network Human Research Ethics Committee approved the study (HREC 18/SCHN/72).
As community seroprevalence was unlikely to be more than 20‐fold higher than the cumulative incidence of notified cases, a sample size of 350 specimens per state and age group (0–4, 5–9, 10–19 years) would be adequate to exclude seroprevalence exceeding 2.0% with 95% confidence. Based on the results of two earlier serosurveys,1,2 we expected that seroprevalence was unlikely to exceed 0.6%. We used the total SARS‐CoV‐2 antibody ELISA (Wantai) we had previously employed in a related national serosurvey.2 Positive specimens were further characterised in immunofluorescent antibody and microneutralisation assays (each: positive titre: ≥ 10).3 We estimated seroprevalence in Bayesian analyses adjusted for test sensitivity and specificity.1 Negative results were reported by email to the parents or carers of participants; positive results, together with clinical advice, were communicated by phone.
Four of 1689 blood specimens were excluded because the children lived outside the five states included in our study. The target sample size (350 children) was achieved for each age group. The median age of participants was 6.7 years (interquartile range, 3.2–11.7 years); 1023 were boys (60.7%), 662 girls (39.3%). Ninety were Indigenous people (5.3%; age‐specific Indigenous population proportion in the 2016 Australian Bureau of Statistics estimated resident population for the five states: 5.4%4) (Box 3). Proportions of tested sera by geographic classification broadly reflected overall population distributions, except that the proportions of New South Wales and Queensland samples from metropolitan areas were larger than the corresponding population proportions; distribution by socio‐economic status (by residence postcode) also matched the population distribution ().
SARS‐CoV‐2‐specific antibodies were detected in seven children (5–9 years, one; 10–19 years, six): three in Victoria, three in New South Wales, one in Queensland; three specimens were also positive in immunofluorescent antibody and microneutralisation assays (Box 3). The crude seroprevalence of SARS‐CoV‐2‐specific antibodies was 0.42% (0–9 years, 0.09%; 10–19 years, 1.07%). After adjusting for ELISA sensitivity and specificity, estimated seroprevalence using the base case prior distribution (uniform probability density between 0.064% and 100%) for people aged 0–19 years was 0.23% (95% credible interval [CrI], 0.06–0.57%). This corresponds to 13 719 (95% CrI, 3579–34 000) people aged 0–19 years in the five mainland states being infected with SARS‐CoV‐2 by early 2021. Using a less conservative prior distribution (greater weight assigned to lower seroprevalence values), estimated seroprevalence was 0.16% (95% CrI, 0.06–0.45%), corresponding to 9545 (95% CrI, 3579–26 841) infections.
We found the prevalence of SARS‐CoV‐2 antibodies in children and adolescents at the beginning of 2021 to be very low, similar to the findings of Australian serosurveys that predominantly sampled adults.1,2 Our opportunistic, consent‐based method of blood sampling was highly effective in achieving the required sample size for analysis and could be used for future sero‐epidemiological monitoring of SARS‐CoV‐2 in children, especially after the surge in infections from mid‐2021.
Box 1 – Notifications of SARS‐CoV‐2 infections, Australia, 13 January 2020 – 31 March 2021*
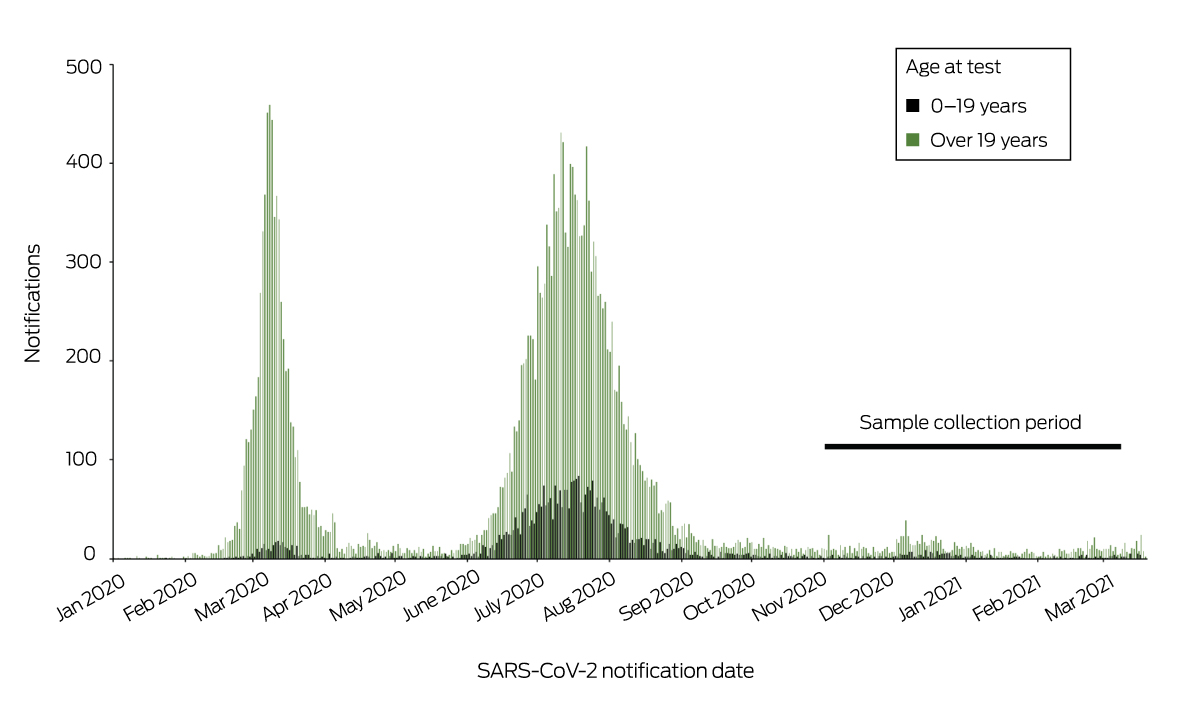
SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2.
* Source: National Interoperable Notifiable Diseases Surveillance System (NINDSS), provided by the Office of Health Protection, Australian Department of Health, on behalf of the Communicable Diseases Network Australia. ◆
Box 2 – Collection of blood specimens from people aged 0–19 years, 3 November 2020 – 12 March 2021, by date and state*
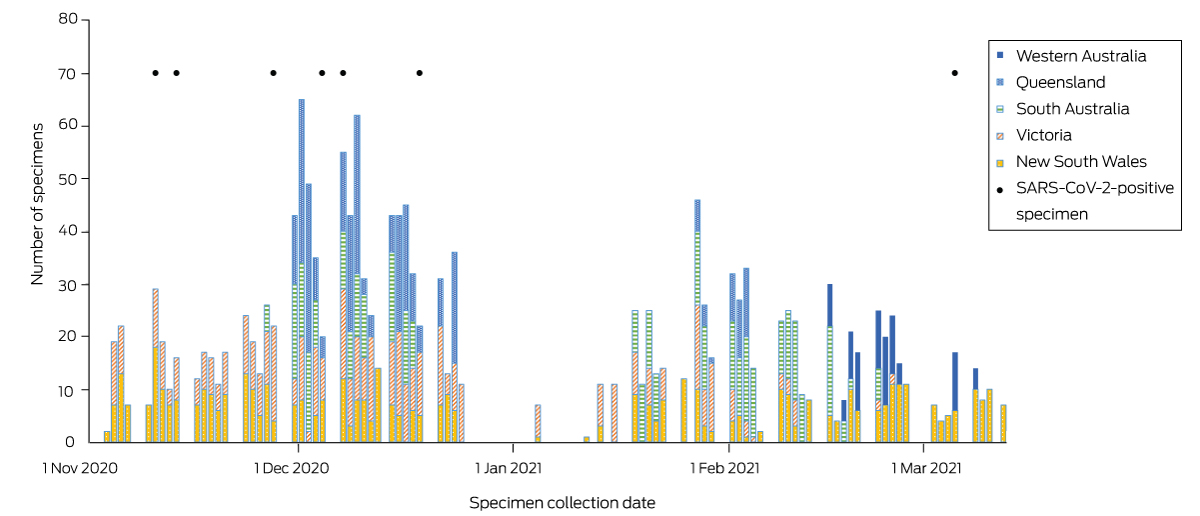
SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2.
* Participating hospitals: Queensland Children’s Hospital, Brisbane (Queensland); Sydney Children’s Hospital Randwick and the Children’s Hospital at Westmead, Sydney (New South Wales); Royal Children’s Hospital and Monash Medical Centre, Melbourne (Victoria); Women’s and Children’s Hospital, Adelaide (South Australia); and the Perth Children’s Hospital, Perth (Western Australia). The positive specimens were collected in Victoria (cases 1, 3, 4), New South Wales (cases 2, 5, 7), and Queensland (case 6). ◆
Box 3 – Blood specimens from people aged 0–19 years, 3 November 2020 – 12 March 2021: outcomes
|
|
|
SARS‐CoV‐2‐positive specimens |
|
||||||||||||
|
Characteristic |
Specimens collected |
SARS‐CoV‐2 antibody ELISA* * |
Immunofluorescent antibody† |
Microneutralisation† |
Crude seroprevalence (ELISA) |
||||||||||
|
|
|||||||||||||||
|
All participants |
1685 |
7 |
3 |
3 |
0.42% |
||||||||||
|
State |
|
|
|
|
|
||||||||||
|
New South Wales |
462 |
3 |
0 |
0 |
0.65% |
||||||||||
|
Victoria |
455 |
3 |
2 |
2 |
0.66% |
||||||||||
|
South Australia |
348 |
0 |
0 |
0 |
— |
||||||||||
|
Queensland |
323 |
1 |
1 |
1 |
0.31% |
||||||||||
|
Western Australia |
97 |
0 |
0 |
0 |
— |
||||||||||
|
Age group (years) |
|
|
|
|
|
||||||||||
|
0–4 |
630 |
0 |
0 |
0 |
— |
||||||||||
|
5–9 |
494 |
1 |
0 |
0 |
0.20% |
||||||||||
|
10–19 |
561 |
6 |
3 |
3 |
1.07% |
||||||||||
|
Residential geographic classification |
|||||||||||||||
|
Major cities |
1306 |
5 |
2 |
2 |
0.36% |
||||||||||
|
Regional |
359 |
2 |
1 |
1 |
0.27% |
||||||||||
|
Remote |
19 |
0 |
0 |
0 |
— |
||||||||||
|
Unknown |
1 |
0 |
0 |
0 |
— |
||||||||||
|
Sex |
|
|
|
|
|
||||||||||
|
Girls |
662 |
1 |
0 |
0 |
0.15% |
||||||||||
|
Boys |
1023 |
6 |
3 |
3 |
0.58% |
||||||||||
|
Indigenous status |
|
|
|
|
|
||||||||||
|
Indigenous |
90 |
0 |
0 |
0 |
— |
||||||||||
|
Non‐Indigenous |
1595 |
7 |
3 |
3 |
0.44% |
||||||||||
|
|
|||||||||||||||
|
ELISA = enzyme‐linked immunosorbent assay; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2. * Positive titre: ≥ 0.9. † Positive titre: ≥ 10. |
|||||||||||||||
Received 1 November 2021, accepted 18 March 2022
- 1. Gidding HF, Machalek DA, Hendry AJ, et al. Seroprevalence of SARS‐CoV‐2‐specific antibodies in Sydney after the first epidemic wave of 2020. Med J Aust 2021; 214: 179‐185. https://www.mja.com.au/journal/2021/214/4/seroprevalence‐sars‐cov‐2‐specific‐antibodies‐sydney‐after‐first‐epidemic‐wave
- 2. Vette KM, Machalek DA, Gidding HF, et al. Seroprevalence of SARS‐CoV‐2‐specific antibodies in Australia following the first epidemic wave in 2020: a national survey. Open Forum Infect Dis 2022; 9: ofac002.
- 3. Hueston L, Kok J, Guibone A, et al. The antibody response to SARS‐CoV‐2 infection. Open Forum Infect Dis 2020; 7: ofaa387.
- 4. Australian Bureau of Statistics. Estimated resident Aboriginal and Torres Strait Islander and non‐Indigenous populations, states and territories; 30 June 2016. 31 Aug 2018. https://www.abs.gov.au/statistics/people/aboriginal‐and‐torres‐strait‐islander‐peoples/estimates‐aboriginal‐and‐torres‐strait‐islander‐australians/jun‐2016/3238055001d001_201608.xls (viewed June 2021).






Open access
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
We acknowledge the support of the Australian Department of Health, which reviewed and provided feedback on our manuscript, and the Snow Foundation, which provided financial support for staff and goods/consumables for the project “SARS‐CoV‐2 serological surveillance in community groups” under Program “BEAT COVID‐19: Urgent research to minimise the health, social and economic impacts of COVID‐19”. We thank Nicole Dinsmore (Children's Hospital at Westmead), who, as PAEDS coordinator, developed the research protocol and coordinated specimen collection, study ethics approval application, and governance. We thank the people involved in registering participant consent and data entry: Maha Benyamin (The Children's Hospital at Westmead), Ajay Jadav, Kathryn Meredith and Laura Rost (National Centre for Immunisation Research and Surveillance), Sonia Dougherty (Queensland Children’s Hospital), Christine Heath (Women’s and Children’s Hospital, Adelaide), Caroline Finucane (Perth’s Children’s Hospital), Karen Bellamy (Monash Medical Centre), Alissa McMunn and Jill Nguyen (Royal Children’s Hospital, Melbourne), Nicole Kerly (Sydney Children’s Hospital), and Elana Forbes (Monash Children’s Hospital). For assistance with serosurvey methodology, sample size calculation and statistical analysis we thank Helen Quinn and Alexandra Hendry (National Centre for Immunisation Research and Surveillance), and Dorothy Machalek and John Kaldor (The Kirby Institute). For pathology processing and testing we thank Dominic Dwyer, Katherine Tudo, William Rawlinson, Zin Naing, and Roy Byun (NSW Pathology), Alison Kesson (The Children’s Hospital at Westmead), Patrick Harris (Pathology Queensland), and Craig Riddle and Geoff Higgins (SA Pathology).
Danielle Wurzel received honoraria by Merck for a presentation not related to this study. Helen Marshall received funding from GlaxoSmithKline, Pfizer, and Sanofi‐Pasteur for investigator‐led and sponsored vaccine trials (paid to the University of Adelaide). Britta von Ungern‐Sternberg has received funding from the Stan Perron Charitable Foundation not related to this study.