Women should be informed about and offered effective contraception as part of abortion care.1 Ovulation can occur as early as eight days after an abortion,2 and more than 50% of women report resuming sexual activity within two weeks.3 Long‐acting reversible contraception (LARC), including implants and intra‐uterine devices (IUDs), can be safely initiated after early medical abortion, and is associated with lower risks of subsequent unintended pregnancies and further abortions than other contraceptive methods.1
We therefore examined hormonal contraception used by women after early medical abortion in Australia, and the influence of contraception type on the likelihood of a further medical abortion. We analysed data in the nationally representative Pharmaceutical Benefits Scheme (PBS) 10% sample4 for women aged 15–49 years for whom mifepristone was dispensed for early medical abortion during 2013–2020.
The primary outcome was hormonal contraception dispensed within 60 days of early medical abortion, including hormonal LARC (hormonal IUDs and implants), depot injections, progestogen‐only pills, and combined oral contraceptive pills (Supporting Information, table 1). We also assessed the relationship between contraception type and the likelihood of another early medical abortion within two years in a Cox proportional hazards model adjusted for age, concession card status, calendar year, dispensing pharmacy location, and prescriber specialty; we report adjusted hazard ratios (aHRs; v no hormonal contraception) with 95% confidence intervals (CIs). The Services Australia External Request Evaluation Committee approved the analysis (reference, RMS0201) and the Monash University Human Research Ethics Committee our study (reference, 22877).
Mifepristone was dispensed to 11 140 women during 2013–2020; hormonal LARC was dispensed within 60 days to 1435 of these women (12.9%) and other forms of hormonal contraception to 1387 women (12.5%). The proportions dispensed hormonal LARC were larger than the overall value for women aged 15–19 years (130 of 673, 19.3%) or 20–24 years (363 of 2441, 14.9%), and also for concession card holders (450 of 2701, 16.7%). Of the 1422 women for whom early medical abortion and contraceptive medicines were prescribed by the same doctor, 756 were dispensed hormonal LARC (53.2%) (Supporting Information, table 2). The proportions of women dispensed LARC were similar in 2013–2014 (116 of 922, 12.6%) and 2019–2020 (512 of 3976, 12.9%); the proportions dispensed other forms of hormonal contraception were 10.7% (99 of 922) in 2013–2014 and 13.1% (521 of 3976) in 2019–2020 (Box 1).
Overall, 594 of the 6570 women for whom two years’ follow‐up data were available (8.3%) were dispensed mifepristone within two years of the index dispensing. Compared with women not dispensed hormonal contraception within 60 days of early medical abortion, the risk of repeat dispensing within 24 months was lower for women dispensed hormonal IUDs (aHR, 0.28; 95% CI, 0.17–0.46) or implants (aHR, 0.39; 95% CI, 0.26–0.57), and greater for women dispensed progestogen‐only pills (aHR, 1.79; 95% CI, 1.09–2.95) (Box 2, Box 3).
Strengths of our study included the nationally representative, high quality PBS dataset, the large sample size, and the long time frame (2013–2020). However, as data on oral contraceptives not listed by the PBS, copper IUDs, and vaginal rings were not available, and contraceptives supplied directly by health clinics or within hospitals are not always included in PBS data, we underestimated total contraception use. Further, we assessed subsequent early medical abortions, but not surgical abortions. Finally, we cannot be certain that dispensed medications were used, or determine whether women changed their contraceptive method during the study period.
During 2012–2020, 25.4% of women who had early medical abortions were dispensed hormonal contraceptive products within 60 days; 12.9% were dispensed hormonal LARC, a considerably smaller proportion than in a 2012 Australian cross‐sectional study of women who had attended Marie Stopes International clinics for medical or surgical abortion (1571 of 6348, 24.7%).5
Our findings indicate that early dispensing of contraception, particularly LARC, is associated with reduced likelihood of repeat early medical abortion. As the proportion of women dispensed LARC was much lower than in other local and overseas studies,1,5 we need to improve understanding, among both health care practitioners and women, of the benefits of immediately adopting reliable contraception after an abortion.
Box 1 – Proportions of women dispensed PBS‐listed hormonal contraceptive items within 60 days of early medical abortion, Australia, 2013–2020, by contraception type and two‐year period
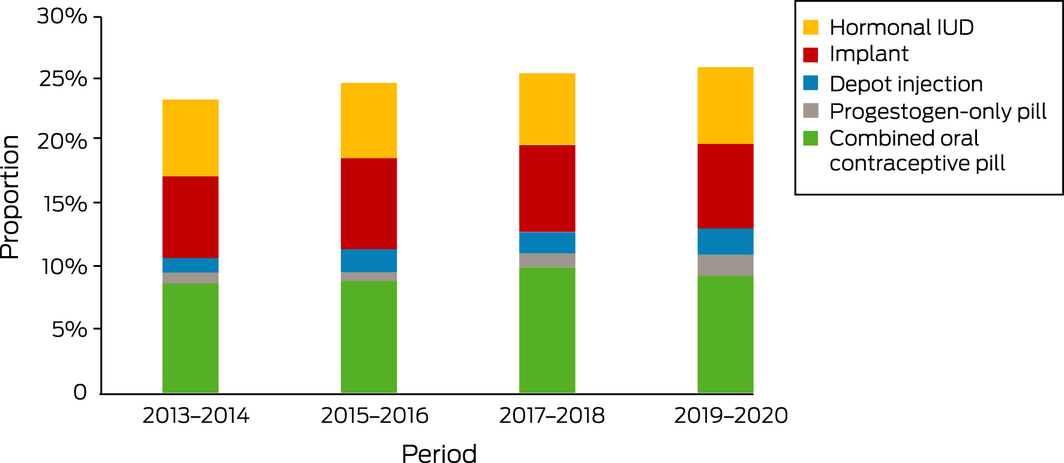
IUD = intra‐uterine device; PBS = Pharmaceutical Benefits Scheme.
Box 2 – Time to repeat early medical abortion, Australia, 2013–2020, by contraceptive type dispensed within 60 days of index early medical abortion: Kaplan–Meier analysis
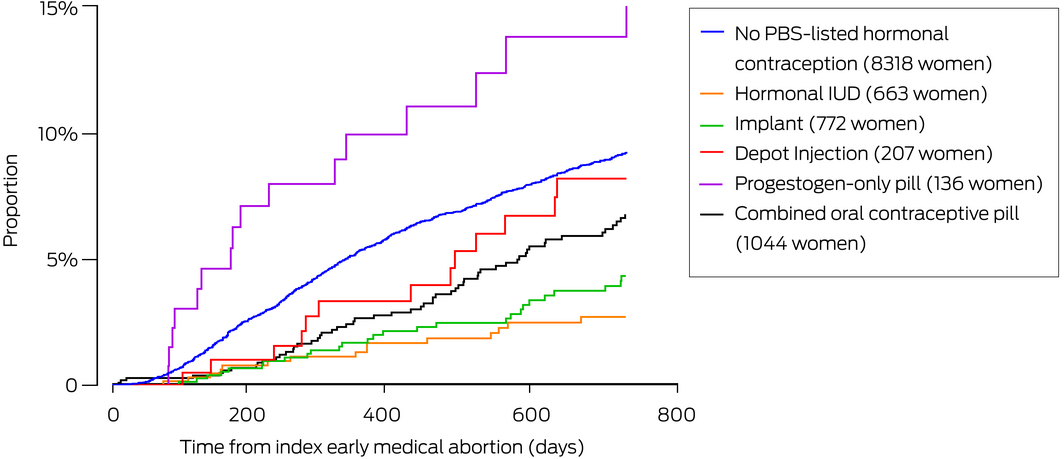
PBS = Pharmaceutical Benefits Scheme.
Box 3 – Contraceptive items dispensed within 60 days of early medical abortion, Australia, 2013–2020, and relative risk of repeat early medical abortion within 24 months
|
Contraception type |
Women |
Repeat early medical abortion |
Adjusted hazard ratio* (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
No PBS contraception |
8318 |
649 (7.8%) |
1 |
||||||||||||
|
Combined oral contraceptive pill |
1044 |
57 (5.5%) |
0.63 (0.48–0.83) |
||||||||||||
|
Progestogen‐only pill |
136 |
16 (12%) |
1.79 (1.09–2.95) |
||||||||||||
|
Depot injection |
207 |
13 (6.3%) |
0.72 (0.41–1.25) |
||||||||||||
|
Implant |
772 |
27 (3.5%) |
0.39 (0.26–0.57) |
||||||||||||
|
Hormonal IUD |
663 |
15 (2.3%) |
0.28 (0.17–0.46) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; IUD = intra‐uterine device; PBS = Pharmaceutical Benefits Scheme. * Adjusted for age, concession card status, calendar year, prescriber specialty, dispensing location. |
|||||||||||||||
Received 21 September 2021, accepted 14 December 2021
- 1. Schmidt‐Hansen M, Hawkins JE, Lord J, et al. Long‐acting reversible contraception immediately after medical abortion: systematic review with meta‐analyses. Hum Reprod Update 2020; 26: 141‐160.
- 2. Schreiber CA, Sober S, Ratcliffe S, Creinin MD. Ovulation resumption after medical abortion with mifepristone and misoprostol. Contraception 2011; 84: 230‐233.
- 3. Boesen HC, Rørbye C, Nørgaard M, Nilas L. Sexual behavior during the first eight weeks after legal termination of pregnancy. Acta Obstet Gynecol Scand 2004; 83: 1189‐1192.
- 4. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Red Notes 2015; 8: 634.
- 5. Goldstone P, Mehta YH, McGeechan K, et al. Factors predicting uptake of long‐acting reversible methods of contraception among women presenting for abortion. Med J Aust 2014; 201: 412‐416. https://www.mja.com.au/journal/2014/201/7/factors‐predicting‐uptake‐long‐acting‐reversible‐methods‐contraception‐among





Luke Grzeskowiak is supported a The Hospital Research Foundation Mid‐Career Fellowship and a Channel 7 Children’s Research Foundation Fellowship. Jenni Ilomäki is supported by Dementia Australia and an Yulgilbar Foundation innovation grant.
Jenni Ilomäki holds a research grant from Amgen and receives consulting fees from AstraZeneca, both of which are unrelated to the research reported in this article.