Atopic dermatitis is characterised by itch (pruritus), characteristic morphology and distribution, a chronic relapsing course, and high personal and social disease burden.1 The risk of concomitant atopic comorbidity (asthma, allergic rhinitis and food allergies) is increased and is highest in early onset and severe atopic dermatitis.2 The prevalence of atopic dermatitis and atopic disease has risen in Western societies in recent decades.2,3
This narrative review provides an overview of the current knowledge on its pathogenesis, which has informed the recommended management of atopic dermatitis and led to new therapeutic options. We searched the PubMed, MEDLINE and Cochrane Library databases for review articles, systematic reviews, meta‐analyses, expert group consensus opinions, and guidelines published between 2016 and 2021. We also included earlier sentinel original articles and key review publications. Where necessary, the authors’ clinical experience is noted.
Clinical aspects of atopic dermatitis
Epidemiology, natural history, burden of disease
Atopic dermatitis affects 20–30% of infants, 15–25% of children and 5–10% of adults.1,4 Its onset is usually in early childhood (in the first year of life in 60%, and by age 5 years in 85% of patients), and in 40–70% it resolves by adolescence.3 A recent Australian study using longitudinal latent class analysis has found that atopic dermatitis persists into adulthood in about 25% of patients.5 Approximately 25% of affected adults have disease onset in adulthood.1,6
Atopic dermatitis has substantial impact on the quality of life of not only affected patients but also their families and caregivers. In addition to disfigurement, itch, skin pain and recurrent infections, there are many comorbid conditions, including respiratory, food and gut allergy; obesity; growth and developmental impairment; chronic sleep deprivation; mental health and behavioural problems; and complications of social withdrawal.1,3 The resulting psychosocial burden of moderate to severe atopic dermatitis is greater than that of type 1 diabetes.7 Personal and societal financial burdens are also substantial.1,3,7
Clinical presentation
Itch, dry skin (xerosis), flexural accentuation (cubital and popliteal fossae), early childhood onset, and personal and family history of atopic disease are hallmarks of atopic dermatitis.1,3 However, atopic dermatitis is heterogenous in its clinical manifestations.8 Infants typically start with scalp, cheek and extensor limb involvement, before developing childhood flexural limb involvement. Adult‐onset atopic dermatitis tends to be localised to flexures, eyelids, face, neck and/or hands, and have fewer associated atopic comorbid conditions (Box 1).1,3,6,9
The presenting features also vary according to the phase of the disease. Acute lesions (ie, arising within 72 hours of onset) are more typical at younger age, are inflammatory, erythematous and oedematous, sometimes with serous exudate; chronic lesions are dry and thickened (lichenified).1,8 In skin of colour, atopic dermatitis can show more papular, follicular, lichenoid, lichenified, prurigo nodularis, and hyperpigmented morphologies, often affecting extensor surfaces.10 Differential diagnoses of atopic dermatitis are presented in Box 2.
Associations and complications
Atopic dermatitis is classically the first manifestation of the atopic march, followed by development of food allergies, asthma and allergic rhinoconjunctivitis.2,3 The proposed mechanism of the atopic march is epicutaneous allergic sensitisation through a defective skin barrier that leads to inflammation (primarily Th2‐driven), progressing to inflammatory processes in distant organs.2,13
Superficial Staphylococcus aureus infection is the most common complication of atopic dermatitis, presenting with acute, sometimes painful, atopic dermatitis exacerbation, associated with honey yellow‐coloured weeping and crusting (impetiginisation) or pustules.14 There is increased susceptibility to viral infections in atopic dermatitis, the most serious being eczema herpeticum due to herpes simplex virus.14 Other viral infections include common warts (human papillomavirus), molluscum contagiosum, and coxsackie enteroviruses causing atypical hand, foot and mouth disease (eczema coxsackium).15
Atopic dermatitis, even if mild, can be associated with significant ocular complications. Potential morbidities include eyelid dermatitis, blepharitis, atopic keratoconjunctivitis, keratoconus, cataract and glaucoma (either primary atopic dermatitis or secondary to topical and systemic corticosteroids).16,17,18
Diagnostic criteria
Atopic dermatitis is a clinical diagnosis without definitive diagnostic tests. Of the diagnostic criteria available, the original and most comprehensive diagnostic standard was developed by Hanifin and Rajka in 1980, which, together with the 1994 UK Working Party’s criteria, is the most extensively validated.17,19,20 Although the definition of “atopic” technically requires IgE‐mediated allergic sensitisation, the terms “atopic dermatitis”, “atopic eczema” and “eczema” are often used synonymously, referring to both the IgE‐mediated allergic sensitised as well as the non‐sensitised forms of the disease.1,21 Skin prick testing and allergen‐specific serum IgE (or radioallergosorbent [RAST]) testing have limited roles in the investigation of atopic dermatitis.
Pathogenesis of atopic dermatitis
The multifactorial pathogenesis of atopic dermatitis comprises impaired epidermal barrier function and immune dysregulation predominantly involving Th2 cytokine inflammatory pathway (Box 3).1,8,22,23,24 There is a complex interaction between barrier dysfunction and inflammation, where one leads to and/or exacerbates the other and vice versa, perpetuating the feedback cycle of chronic inflammation and injury.2 Other contributing elements to atopic dermatitis pathogenesis are genetics, environmental factors, S. aureus colonisation or infection, and neurogenic inflammation.1,8,22,23,24
Skin barrier function
The loss of function mutation in the filaggrin (FLG) gene (filament aggregating protein) (Box 3) is of major pathogenic significance in atopic dermatitis and other atopic conditions. FLG encodes a key protein in the stratum corneum (outer layer of the epidermis) that is critical for skin barrier integrity.13 FLG deficiency is associated with early onset, more severe and persistent atopic dermatitis. However, other genetic and environmental modifiers are involved in the pathogenesis of atopic dermatitis, as only 40% of FLG heterozygotes develop atopic dermatitis.13 Additionally, FLG deficiency is present in only up to one‐third of patients, is not associated with adult‐onset atopic dermatitis, and differs between ethnic groups.1,6 There are dozens of genes involved in atopic dermatitis, such as those encoding other proteins of the epidermal barrier and immune signalling pathways.1,13
Immune dysregulation
A skew towards the Th2 pathway (Box 3) is consistently seen in atopic dermatitis especially in its acute phase (mediated by interleukin [IL]‐4, IL‐5, IL‐13 and IL‐31 cytokines), together with Th22 upregulation (IL‐22). Additionally, Th1 (IL‐12, IFN‐γ) and Th17 (IL‐17) activation are variably observed. IL‐31 and substance P (neurokinin NK‐1 receptor) are key itch neuromodulators. The Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway is implicated in atopic dermatitis, mediating the production of pro‐inflammatory cytokines along the Th2, Th1 and Th17 pathways. Phosphodiesterase type 4 (PDE4) activity is elevated in atopic dermatitis, which increases pro‐inflammatory cytokines through degradation of cyclic adenosine monophosphate.1,8,22,23,24,25
Cutaneous microbiome imbalance
There is increased S. aureus adherence and/or proliferation, and reduction of overall microbial diversity in atopic dermatitis skin compared with the more diverse commensal bacterial population of normal skin flora. S. aureus abundance correlates with flares and severity of atopic dermatitis.1,14
Management of atopic dermatitis
Overview and general measures
Education and psychosocial support of patients and carers is critical for optimal treatment outcomes. Atopic dermatitis management is multifaceted, involving a stepwise approach, from basic skin care with regular emollient for maintenance and prevention of flares to additional specific treatment of active atopic dermatitis, depending on its degree of severity. Atopic dermatitis distribution and morphology, disease impact, personal preferences, comorbid conditions, psychosocial factors, past therapies, and treatment accessibility all affect treatment choice, tailored to individual needs (Box 4). If relevant, any exacerbating environmental factors, such as extremes of temperature and humidity, irritants and allergens, alkaline detergents, and abrasive clothing, should be addressed.18,29
Topical corticosteroids are usually the first line anti‐inflammatory medication for mild to moderate atopic dermatitis, with topical calcineurin inhibitors or crisaborole as alternative options for face and flexures. For moderate to severe atopic dermatitis, treatment intensity is usually increased by stepwise progression: from higher potency of topical corticosteroid to phototherapy, dupilumab, and systemic immunosuppression.
The food allergy–atopic dermatitis relationship is complex and is beyond the scope of this review. Food elimination diets are not a general recommendation for patients with atopic dermatitis. Food allergy testing should be pursued only in young children with moderate to severe atopic dermatitis in whom standard skin‐directed treatments have failed, or where appropriately indicated by post‐exposure symptoms, under the supervision of an allergist and nutritionist.18,29,30
Clinical indicators of hypersensitivity reactions may warrant skin prick or allergen‐specific IgE (type I) or patch (type IV) testing. Examples of type I hypersensitivity are food and airborne allergy, which may be suggested respectively by post‐prandial flares or unexplained atopic dermatitis instability (usually in childhood) and airborne‐affected skin accentuation. Contact (type IV) allergy may both mimic and arise in atopic dermatitis (including to emollients and topical treatments).18,29
Emollient moisturisers
Liberal daily or twice daily all‐over moisturisation is the cornerstone of atopic dermatitis management to restore skin barrier function and thus reduce inflammation.18,31 It has demonstrated efficacy in secondary prevention, reducing flare frequency and time to recurrence, and less topical corticosteroid required for atopic dermatitis control.32 Emollient application to wet skin immediately after a lukewarm shower or bath (“soak and smear”) optimises its impact. Occlusion with wet wraps or dressings further enhances skin hydration but also potential corticosteroid absorption.18,31
Pilot studies suggested an effect of regular emollient in the primary prevention of atopic dermatitis in high risk infants. However, larger randomised control trials have subsequently failed to show any benefit.1,33,34,35 Further research is currently underway.36
Moisturisers come in a plethora of formulations and include a combination of ingredients, grouped into:
- occlusives, which provide barrier protection against external injury and reduce transepidermal water loss (ointments, water‐in‐oil creams);
- humectants, which provide hydration by attracting and binding water (glycerol and natural moisturising factors such as urea, lactate, amino acids, pyrrolidone carboxylic acid, hyaluronic acid); and
- therapeutic ingredients that improve skin barrier, hydrate, reduce inflammation and restore lipids (eg, ceramides, plant oils rich in linoleic acid, antioxidants, natural moisturising factors, colloidal oatmeal, and acidic buffers such as citric acid, optimising skin pH to 4–5).37
No single moisturiser has been found to be superior to another.32 Inert water‐free oils (namely, petrolatum and white soft and liquid paraffin) are recommended, being preservative‐free (thus allergen‐free) and more efficacious as barrier moisturisers than watery lotions.31 Ointments also sting less if applied on broken skin, but are disadvantageous because they leave an oily surface shine and can cause follicular occlusion, particularly on hair‐bearing skin in adults. Moisturiser choice depends on site, atopic dermatitis lesional factors, and patient preference. Products containing excessive ingredients or common allergens (fragrances, undesirable preservatives) and costly options with no proven benefit are discouraged.31 The use of food‐based products as emollients (eg, goat milk, wheat, oat, peanut oil) are also not recommended as there is evidence that cutaneous sensitisation via inflamed skin increases the risk of food allergies.38,39,40
Topical corticosteroids
Following on from emollient barrier restoration, topical corticosteroids are the first line pharmacological management of atopic dermatitis, showing proven efficacy and safety.1 Mild atopic dermatitis can resolve with emollients alone, but topical anti‐inflammatory therapies are usually required to downregulate inflammatory activity in order to break the cycle of recurrent flare‐ups. The recommended potency of topical corticosteroids depends on the severity and location of the atopic dermatitis: low/mild potency for face and flexures (axilla, inframammary, inguinal, cubital/popliteal fossa, genitals) and high potency for thickened lichenified areas, palms and soles.31 Commonly available topical corticosteroids in Australia and their relative potencies are shown in Box 5.41 Education of patients and carers is paramount, as inadequate application of topical therapy is the typical reason for suboptimal response to treatment.41 In particular, “steroid phobia” needs to be addressed to ensure adherence to therapy and therefore disease control.31,41 Authority prescriptions for increased quantities of topical corticosteroids under the Australian Pharmaceutical Benefits Scheme (PBS) are often required if atopic dermatitis is extensive. Occlusion of topical corticosteroids can enhance penetration and efficacy for severe, lichenified atopic dermatitis; however, inappropriate long term use of large quantities of potent topical corticosteroids over large surface areas can cause systemic absorption, apart from local atrophy at the site of application.31,42 Patient education and supervision of therapy is therefore important.29 Emollients should be used in combination with topical corticosteroids, as they have greater efficacy when used together compared with topical corticosteroids alone.32 Daily therapy should be continued until active lesions are completely cleared to minimise the likelihood of rebound recurrence, and once clear, tapering to cessation is recommended.18 Patients who have regular flare‐ups may require intermittent proactive use as maintenance prophylaxis.29
Topical calcineurin inhibitors
Calcineurin inhibitors suppress T cell activation and downregulate the secretion of pro‐inflammatory mediators.31 Topical pimecrolimus and tacrolimus are non‐steroidal anti‐inflammatories used in particular for flexures and the face, especially on and around eyelids, to avoid skin atrophy and other facial complications of potent topical corticosteroids.18,31,43 Pimecrolimus 1% cream, available on the PBS, is equivalent to mild potency topical corticosteroids and is useful for eyelids and mild facial atopic dermatitis. Tacrolimus 0.03–0.1% ointment is currently available mostly by compounding in Australia, with a potency considered equivalent to moderate to potent topical corticosteroids. Disadvantages of topical calcineurin inhibitors are cost and the most common side effect of transient burning or itch at the application site.44
Phosphodiesterase type 4 inhibitors
Crisaborole 2% ointment, a topical PDE4 inhibitor, has been shown to be effective and well tolerated and was approved by the Australian Therapeutic Goods Administration (TGA) in 2019 for mild to moderate atopic dermatitis.45 It is not currently subsidised by the PBS. Similar to topical calcineurin inhibitors, the most common side effect of crisaborole is transient localised stinging.23
Antimicrobial treatment
Atopic dermatitis‐affected skin is commonly colonised by S. aureus so a positive culture from a swab alone does not distinguish colonisation from active infection.46 The decision to treat with systemic antibiotics is based on clinical significance, ideally with antimicrobial sensitivities confirmed on culture.14 Topical antibiotics are not recommended for use on an as‐needed basis, particularly as bacterial resistance is an increasing clinical challenge.31
Diluted bleach baths are beneficial in atopic dermatitis by multiple possible mechanisms: bacterial decolonisation, increased skin hydration, altered skin pH and anti‐inflammatory activity.31,47 Daily 10‐minute tepid diluted bleach baths for acute infected flares of atopic dermatitis or twice weekly for ongoing maintenance may be recommended. An example of appropriate dilution is 12 mL unfragranced regular household bleach (containing about 4.2% sodium hypochlorite) per 10 L water for 0.005% concentration.15,18 It is important that baths are followed by liberal moisturisation.18 Intranasal mupirocin used together with bleach baths can help reduce S. aureus colonisation in patients and household members, especially for recurrently infected atopic dermatitis.31 Alternatively, 0.006% sodium hypochlorite body wash has also shown improved outcomes for moderate to severe S. aureus‐colonised atopic dermatitis without necessarily reducing S. aureus, suggesting beneficial effects other than antimicrobial action.47
Antihistamines
Oral antihistamines (preferably non‐sedating) are rarely useful for atopic dermatitis itch, unless there is coexisting dermographism, urticaria or other atopic conditions such as rhinoconjunctivitis. Antihistamines should not be used routinely or instead of topical therapies. Prolonged sedating antihistamines use may incur neurodevelopmental complications.18,46,48,49
Phototherapy
Natural sunlight or dermatologist‐administered phototherapy can be beneficial in atopic dermatitis through inhibition of antigen‐presenting Langerhans cells and keratinocyte cytokine production.18 However, phototherapy can also be deleterious in a subset of patients, including those with severe, acute atopic dermatitis and those in whom heat triggers sweating and itch.46
Dupilumab
Most patients with atopic dermatitis can be adequately managed with education, trigger avoidance, emollients, topical anti‐inflammatory agents, and phototherapy. Some moderate to severe atopic dermatitis requires additional systemic immunosuppressive or biologic therapy.26,28 Recently, dupilumab has become the first available targeted systemic therapy for atopic dermatitis with superior efficacy and safety profiles compared with existing non‐targeted immunosuppressive agents.50,51 In Australia, dupilumab was TGA‐approved in 2018 and has been PBS‐available since March 2021 for patients aged 12 years and older with chronic severe atopic dermatitis that has failed potent topical corticosteroid therapy. Administered as a fortnightly subcutaneous injection, dupilumab is a recombinant fully human monoclonal antibody inhibiting IL‐4 receptor α that leads downstream inhibition of IL‐4/IL‐13‐mediated Th2 signalling.
Non‐infectious conjunctivitis is its most common adverse event, occurring in 26% of patients, the mechanism of which remains unclear.51 Prophylactic lubricating eye drops are recommended from the time of commencement of dupilumab, especially if atopic ocular symptoms exist.12,51 Th2 immunity is critical in host defence against parasites, in particular helminths, and although trials have not shown an increase in parasitic infections with dupilumab, pre‐treatment screening (eg, Strongyloides serology) is to be considered, especially if there are risk factors or endemic disease.52
Systemic corticosteroids
Systemic corticosteroids are not recommended in the long term management of atopic dermatitis, as rebound flares occur on cessation and significant metabolic adverse effects follow prolonged use.53 Oral prednisolone may rarely have a role in atopic dermatitis as rescue treatment for acute severe flares because of its dose‐dependent and rapid efficacy.45,54 Its use should be infrequent, limited to short courses (up to 2 weeks), and only in conjunction with intensive topical therapy and while potentially bridging to alternative long term immunomodulatory therapy.52,54
Systemic immunomodulatory treatments
Before the recent availability of dupilumab, traditional immunosuppressive or immunomodulatory agents such as azathioprine, methotrexate, mycophenolate mofetil, cyclosporin were more commonly used as steroid‐sparing agents in the management of moderate to severe chronic atopic dermatitis. Myelosuppression, infection and carcinogenesis are potential serious adverse effects common to these medications, complications largely related to dose and duration of use. In addition, hepatotoxicity is an issue with methotrexate and azathioprine, while hypertension and renal impairment are complications of cyclosporin.15,45,55
Future targeted therapies in the pipeline
Janus kinase inhibitors
Studies have increasingly demonstrated the efficacy of topical and oral JAK inhibitors in atopic dermatitis — topical and oral tofacitinib (JAK1/3), oral baricitinib (JAK1/2), oral abrocitinib (JAK1), oral upadacitinib (JAK1), topical ruxolitinib (JAK1/2) and topical delgocitinib (pan‐JAK).25,56 Oral baricitinib and upadacitinib were approved by the TGA in 2021, and upadacitinib has been available in the PBS since February 2022 for the treatment of moderate to severe atopic dermatitis in patients aged 12 years or older in whom systemic therapy is indicated. The adverse effects of JAK inhibitors are potentially serious, including opportunistic infections, immunosuppression‐associated malignancy, myelosuppression, venous thromboembolism, and cardiovascular events, although risks may vary between agents.25 Notwithstanding these potential complications, the benefits of JAK inhibitors in controlling severe atopic dermatitis may outweigh their risks in a subset of patients.
Targeted agents undergoing development
There are multiple targeted agents undergoing clinical trials for atopic dermatitis. Agents that have shown promising efficacy include inhibitors of JAK, JAK/spleen tyrosine kinase, IL‐13 (lebrikizumab and tralokinumab), IL‐31 (nemolizumab), and IL‐22 (fezakinumab). Antagonists of IL‐12/23p40 of Th17 pathway (ustekinumab), IL‐17A (secukinumab), IgE (omalizumab), and IL‐5 (mepolizumab) have not been found to be effective. Others undergoing investigation are inhibitors of thymic stromal lymphopoietin (tezepelumab), NK‐1 binding substance P (serlopitant), IL‐33, histamine 4 receptor, and PDE4 (apremilast).23,24,25,50,55,56
Conclusion
Atopic dermatitis is the commonest chronic inflammatory skin condition and causes significant physical and psychosocial morbidity. The heterogeneity of atopic dermatitis requires the tailoring of its multifaceted management, which involves avoidance of environmental triggers, restoration of skin barrier function, management of S. aureus superinfection, use of anti‐inflammatory medications, and management of comorbid conditions. The treatment of severe atopic dermatitis has progressed with the current availability of safer and effective targeted systemic immunomodulatory agents such as dupilumab and JAK inhibitors. Ongoing elucidation of genetic and inflammatory pathways responsible for atopic dermatitis holds promise for new targeted treatments and increasingly personalised atopic dermatitis therapy.
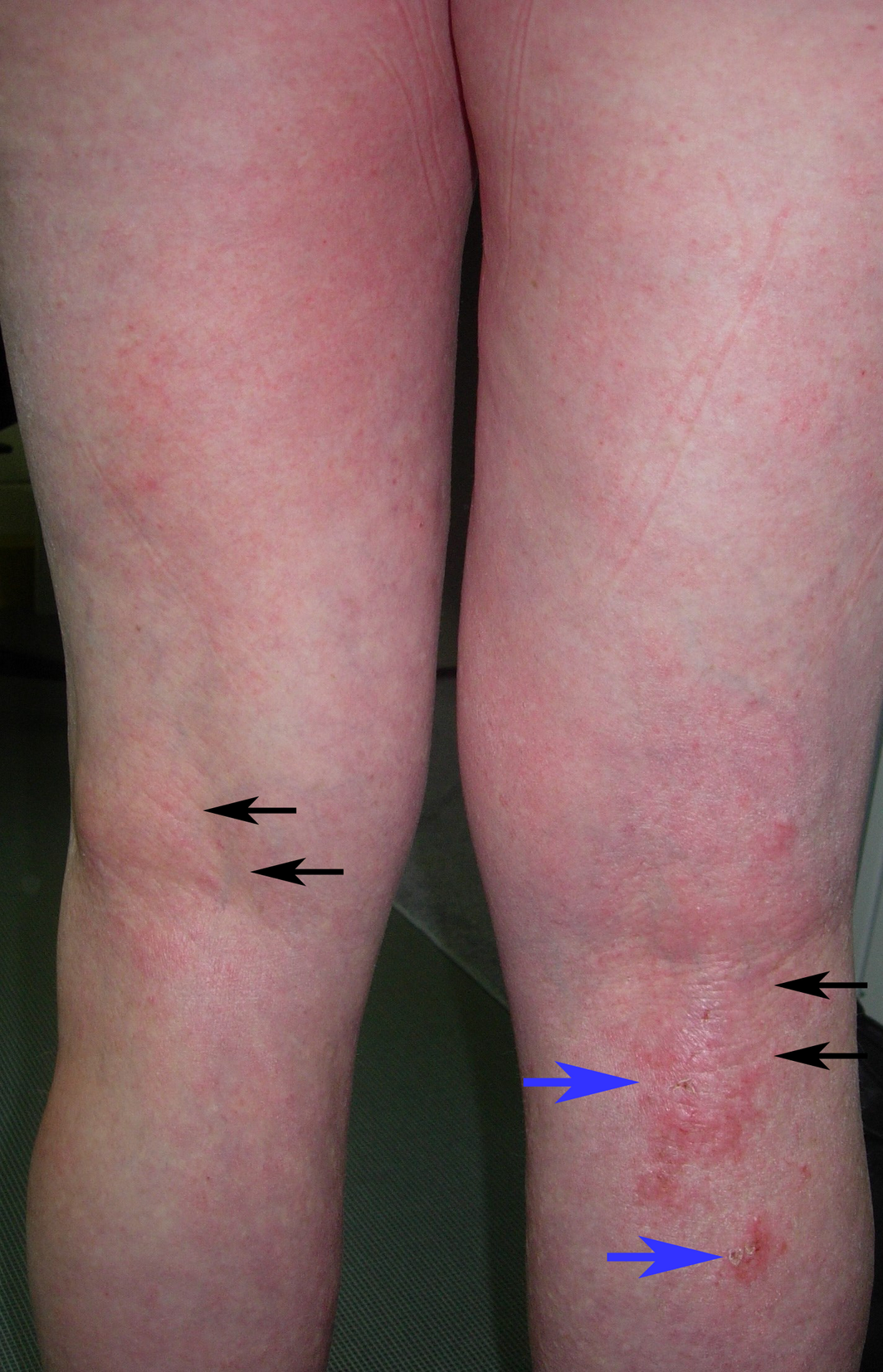
Box 1 – Posterior lower limbs in an adult with generalised atopic dermatitis: diffuse patchy erythema with lichenification (thickening) from chronic scratching (black arrows), and foci of crusted erosions (blue arrows)
Box 2 – Differential diagnoses of atopic dermatitis1,6,11,12
|
Age |
Differential diagnoses |
||||||||||||||
|
|
|||||||||||||||
|
Infancy |
|
||||||||||||||
|
Childhood |
|
||||||||||||||
|
Adolescence and adulthood |
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 – Immunopathogenesis of atopic dermatitis1,8,22,23,24
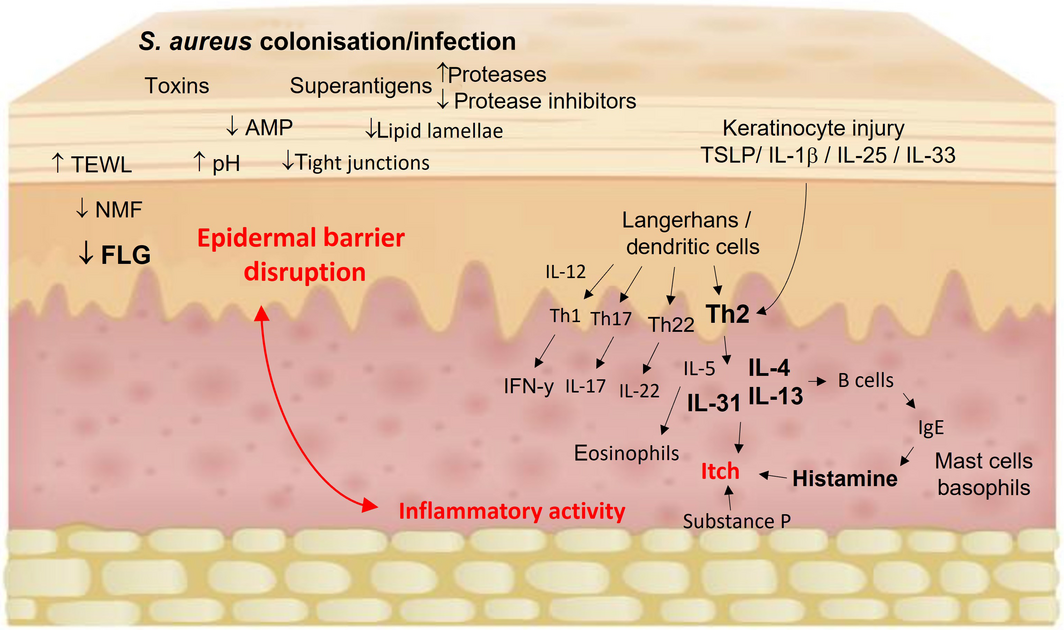
AMP = antimicrobial peptides; FLG = filaggrin; IFN = interferon; IL = interleukin; NMF = natural moisturising factors; TEWL = transepidermal water loss; TSLP = thymic stromal lymphopoietin. A complex interplay between epidermal barrier dysfunction, immune dysregulation with skewing towards the Th2 pathway (IL‐4, IL‐5, IL‐13, IL‐31) and Th22 upregulation (IL‐22), altered skin microbiome with Staphylococcus aureus colonisation and/or infection, and itch. Components contributing to epidermal barrier function are FLG, other epidermal barrier proteins (keratins, loricrin, involucrin, plakins), intercellular lipid matrix (ceramides, cholesterol, free fatty acids), intercellular tight junctions, protease activity, endogenous AMP, NMFs (degradation products of FLG), and acidic skin pH. Barrier dysfunction leads to increased TEWL, allergen exposure and susceptibility to microbial colonisation and infection (S. aureus toxins and superantigens) that ultimately trigger the cytokine cascade. Environmental stimuli (eg, scratching and infection) lead to keratinocyte release of chemokines and alarmins, including TSLP, IL‐1β, IL‐25 and IL‐33 that activate Th2 cells. In addition to contributing further to barrier dysfunction, Th2 cytokines recruit eosinophils and stimulate B cells leading to IgE class switching and downstream mast cell release of histamine. IL‐31 and substance P (NK‐1 receptor) are key neuromodulators of itch. IL‐22 is involved in epidermal hyperproliferation in chronic lichenification. Th1 and Th17 pathways have some role in chronic atopic dermatitis with differences depending on age and ethnicity.
Box 4 – Stepwise management of atopic dermatitis: baseline maintenance with increasing intensity of treatment according to severity of disease. Progression is also affected by disease impact, personal preferences, comorbid conditions, psychosocial factors, past therapies, and treatment accessibility12,26,27,28
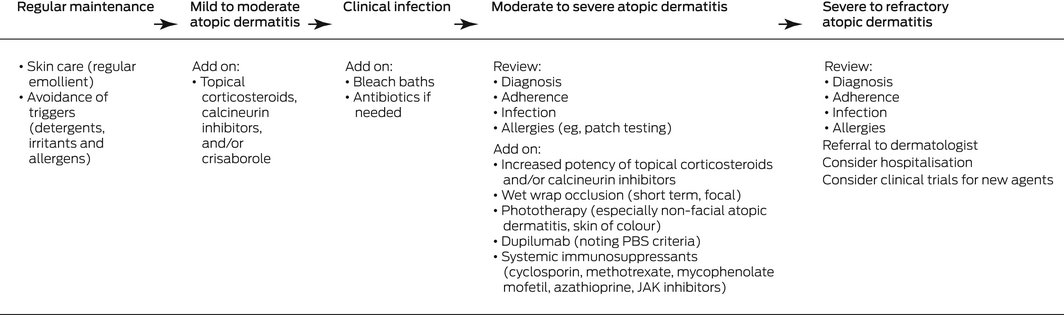
JAK = Janus kinase; PBS = Pharmaceutical Benefits Scheme.
Box 5 – Topical corticosteroid preparations and potency classification41
|
Potency |
Drug |
||||||||||||||
|
|
|||||||||||||||
|
Low/mild |
|
||||||||||||||
|
Moderate |
|
||||||||||||||
|
Potent |
|
||||||||||||||
|
Very potent |
|
||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Provenance: Commissioned; externally peer reviewed.
- Michelle SY Goh1,2
- Jenny SW Yun1,3
- John C Su4,5
- 1 Peter MacCallum Cancer Centre, Melbourne, VIC
- 2 St Vincent’s Hospital Melbourne, Melbourne, VIC
- 3 Royal Melbourne Hospital, Melbourne, VIC
- 4 Eastern Health, Monash University, Melbourne, VIC
- 5 Murdoch Children’s Research Institute, Melbourne, VIC
John Su has been a consultant/speaker/investigator for AbbVie, Amgen, Bioderma, Bristol Myers Squibb, Ego Pharmaceuticals, Eli‐Lilly, Janssen, LEO Pharma, L’Oreal, Mayne, Novartis, Pfizer, Pierre‐Fabre, and Sanofi.
- 1. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345‐360.
- 2. Lowe AJ, Leung DYM, Tang MLK, et al. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol 2018; 120: 145‐151.
- 3. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003; 112: S118‐S127.
- 4. Martin PE, Koplin JJ, Eckert JK, et al. The prevalence and socio‐dermographic risk factors of clinical eczema in infancy: a population‐based observational study. Clin Exp Allergy 2013; 43: 642‐651.
- 5. Lopez DJ, Lodge CJ, Bui DS, et al. Establishing subclasses of childhood eczema, their risk factors and prognosis. Clin Exp Allergy 2022; https://doi.org/10.1111/cea.14139 [Epub ahead of print].
- 6. Vakharia PP, Silverberg JI. Adult‐onset atopic dermatitis: characteristics and management. Am J Clin Dermatol 2019; 20: 771‐779.
- 7. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child 1997; 76: 159‐162.
- 8. Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol 2019; 143: 1‐11.
- 9. Chan OB, Dharmage SC, Zeleke B, et al. Adult‐onset atopic dermatitis in a middle aged Australian population: a population‐based cohort study [poster]. 11th George Rajka International Symposium on Atopic Dermatitis, 19–20 April 2021; Seoul, Republic of Korea. Acta Derm Venereol 2021; 101. https://doi.org/10.2340/00015555‐3793.
- 10. Zhao CY, Hao EY, Oh DD, et al. A comparison study of clinician‐rated atopic dermatitis outcome measures for intermediate‐ to dark‐skinned patients. Br J Dermatol 2017; 176: 985‐992.
- 11. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis. Section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338‐351.
- 12. Narla S, Silverberg JI, Simpson EL. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol 2022; 86: 628‐636.
- 13. Irvine AD, McLean IWH, Leung DYM. Filaggrin mutations associated with skin and allergic disease. N Engl J Med 2011; 365: 1315‐1327.
- 14. Alexander H, Paller AS, Traidl‐Hoffmann C, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol 2020; 182: 1331‐1342.
- 15. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850‐878.
- 16. Carmi E, Defossez‐Tribout C, Ganry O, et al. Ocular complications of atopic dermatitis in children. Acta Derm Venereol 2006; 86: 515‐517.
- 17. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; 92 (Suppl): 44‐47.
- 18. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657‐682.
- 19. Williams HC, Burney PG, Hay RJ, et al. The UK Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994; 131: 383‐396.
- 20. Brenninkmeijer EEA, Schram ME, Leeflang MMG, et al. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 2008; 158: 754‐765.
- 21. Silverberg JI, Thyssen JP, Paller AS, et al. What’s in a name? Atopic dermatitis or atopic eczema, but not eczema alone. Allergy 2017; 72: 2026‐2030.
- 22. Brunner PM, Leung DYM, Guttman‐Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 120: 34‐41.
- 23. Puar N, Chovatiya R, Paller A. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol 2021; 126: 21‐31.
- 24. Renert‐Yuval Y, Guttman‐Yasky E. New treatments for atopic dermatitis targeting beyond IL‐4/IL‐13. Ann Allergy Asthma Immunol 2020; 124: 28‐35.
- 25. He H, Guttman‐Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol 2019; 20: 181‐192.
- 26. Simpson EL, Bruin‐Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017; 77: 623‐633.
- 27. Boguniewicz M, Fonacier L, Guttman‐Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol 2018; 120: 10‐22.
- 28. Fishbein AB, Silverberg JI, Wilson EJ, et al. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract 2020; 8: 91‐101.
- 29. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis. Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014; 71: 1218‐1233.
- 30. Eigenmann PA, Beyer K, Lack G, et al. Are avoidance diets still warranted in children with atopic dermatitis? Pediatr Allergy Immunol 2020; 31: 19‐26.
- 31. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116‐132.
- 32. Van Zuuren EJ, Fedorowicz Z, Christensen R, et al. Emollients and moisturisers for eczema. Cochrane Database Syst Rev 2017; (2): CD012119.
- 33. Kelleher MM, Cro S, Cornelius V, et al. Skincare interventions in infants for preventing eczema and food allergy. Cochrane Database Syst Rev 2020; (2): CD013534.
- 34. Skjerven HO, Rehbinder EM, Vettukattil R et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster‐randomised trial. Lancet 2020; 395: 951‐961.
- 35. Chalmers JR, Haines RH, Bradshaw LE et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised control trial. Lancet 2020; 395: 962‐972.
- 36. Lowe A, Su J, Tang M et al. PEBBLES study protocol: a randomised controlled trial to prevent atopic dermatitis, food allergy and sensitisation in infants with a family history of allergic disease using a skin barrier improvement strategy. BMJ Open 2019; 9: e024594.
- 37. Hebert AA, Rippke F, Weber TM, et al. Efficacy of nonprescription moisturizers for atopic dermatitis: an updated review of clinical evidence. Am J Clin Dermatol 2020; 21: 641‐655.
- 38. Lack, G. Update on risk factors for food allergy. J Allergy Clin Immunol 2012; 129: 1187‐1197.
- 39. Voskamp AL, Zubrinich CL, Abramovitch JB, et al. Goat’s cheese anaphylaxis after cutaneous sensitization by moisturizer that contained goat’s milk. J Allergy Clin Immunol Pract 2014; 2: 629‐630.
- 40. Kobayashi T, Ito T, Kawakami H, et al. Eighteen cases of wheat allergy and wheat‐dependent exercise‐induced urticarial/anaphylaxis sensitized by hydrolyzed wheat protein in soap. Int J Dermatol 2015; 54: e302‐305
- 41. Mooney E, Rademaker M, Dailey R, et al. Adverse effects of topical corticosteroids in paediatric eczema: Australasian consensus statement. Australas J Dermatol 2015; 56: 241‐251.
- 42. González‐López G, Ceballos‐Rodríguez RM, González‐López JJ. Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and meta‐analysis. Br J Dermatol 2017; 177: 688‐695.
- 43. Siegfried EC, Jaworski JC, Kaiser JD, et al. Systematic review of published trials: long‐term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatrics 2016; 16: 75.
- 44. Martins JC, Martins C, Aoki V, et al. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev 2015; (7): CD009864.
- 45. Schlessinger J, Shepard JS, Gower R, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild‐to‐moderate atopic dermatitis: a phase IV open‐label study (CrisADe CARE 1). Am J Clin Dermatol 2020; 21: 272‐284.
- 46. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis. Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327‐349.
- 47. Majewski S, Bhattacharya T, Asztalos M, et al. Sodium hypochlorite body wash in the management of Staphylococcus aureus‐colonized moderate‐to‐severe atopic dermatitis in infants, children, and adolescents. Pediatr Dermatol 2019; 36: 442‐447.
- 48. Matterne U, Böhmer MM, Weisshar E, et al. Oral H1 antihistamines as ‘add‐on’ therapy to topical treatment for eczema. Cochrane Database Syst Rev 2019; (1): CD012167.
- 49. Fitzsimons R, van der Poel L, Thronhill W, et al. Antihistamine use in children. Arch Dis Child Educ Pract Ed 2015; 100: 122‐131.
- 50. Sawangjit R, Dilokthornsakul P, Lloyd‐Lavery A, et al. Systemic treatments for eczema: a network meta‐analysis. Cochrane Database Syst Rev 2020; (9): CD013206.
- 51. Halling A, Loft N, Silverberg JI, et al. Real‐world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta‐analysis. J Am Acad Dermatol 2021; 84: 139‐147.
- 52. Eichenfield LF, Bieber T, Beck LA, et al. Infections in Dupilumab clinical trials in atopic dermatitis: A Comprehensive Pooled Analysis. Am J Clin Dermatol 2019; 20: 443‐456.
- 53. Drucker AM, Eyerich K, de Bruin‐Weller MS, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018; 178: 768‐775.
- 54. Rademaker M, Agnew K, Andrews M, et al. Managing atopic dermatitis with systemic therapies in adults and adolescents: An Australian/New Zealand narrative. Australas J Dermatol 2020; 61: 9‐22.
- 55. Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta‐analysis. Allergy 2020; 76: 1053‐1076.
- 56. Chapman S, Kwa M, Gold LS, Lim HW. Janus kinase inhibitors in dermatology: Part I. A comprehensive review. J Am Acad Dermatol 2022; 86: 406‐413.





Summary