To the Editor: The average woman giving birth in Australia has ten to 12 antenatal visits and a 2–4 days inpatient stay, representing 8 months of intense engagement with health services. In 2020, women in Melbourne endured a prolonged lockdown period due to the coronavirus disease 2019 (COVID‐19) pandemic.1 During this time, the maternity sector had to move quickly to address three urgent priorities.
Firstly, all 12 public maternity hospitals in Melbourne joined forces to create the Collaborative Maternity and Newborn Dashboard for the COVID‐19 pandemic (CoMaND) to meet the need for timely perinatal data collection.2 By harnessing hospital maternity data collection systems under a research protocol, they could centrally monitor perinatal outcomes to assess indirect impacts of the sector’s pandemic response (Box).
The second of the priorities was to institute a system to record outcomes for women who were infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) during pregnancy. To this end, the Coronavirus Health Outcomes in Pregnancy and Newborns (CHOPAN) registry was established. It has collected information from 100 women with confirmed SARS‐CoV‐2 infection during pregnancy and has since expanded nationally (https://www.psanz.com.au/covid-19/).
The third priority was to address the exclusion of pregnant women from COVID‐19 treatment trials, which occurred despite the fact that many of the investigational drugs had established pregnancy safety profiles.4 The Australasian COVID‐19 Trial (ASCOT) is an international multicentre randomised adaptive platform clinical trial of COVID‐19 therapies (https://www.ascot‐trial.edu.au). After representations from the maternity sector, a pregnancy ASCOT working group tasked with facilitating the safe inclusion of pregnant women was appointed, which established a welcome precedent for inclusion of pregnant women in future clinical research.5
The CoMaND and CHOPAN collaborations are exemplars of clinician‐led initiatives for data‐informed emergency responses in maternity care. It is anticipated that these resources will be of ongoing value into the COVID‐19 vaccination era. Successful advocacy for the inclusion of pregnant women in clinical trials may be another positive legacy of the COVID‐19 pandemic. Their safe inclusion in clinical trials takes us a step closer to an equitable health service, ensuring we generate a robust evidence base to direct clinical care for pregnant women and their children.
Box – An example of outcome reporting from the fifth CoMaND report2
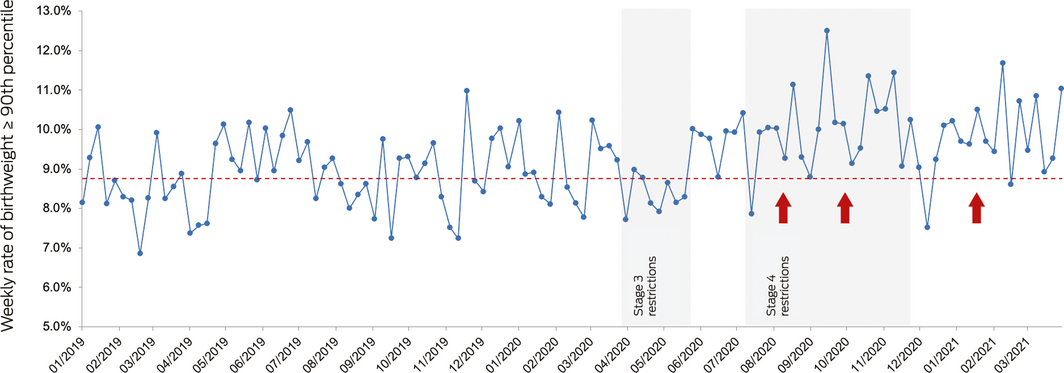
Denominator: number of singleton babies at ≥ 20 weeks’ gestation. Numerator: number of babies who meet the denominator criteria with birth weight ≥ 90th percentile adjusted for fetal sex and gestational age. Pre‐pandemic median: 8.75%. Significant shifts (≥ 6 weeks above the pre‐pandemic median) indicated with red arrows. Percentile source: Dobbins et al.3
- 1. Thomas H, Angrist N, Cameron‐Blake E, et al. Oxford COVID‐19 government response tracker. Blavatnik School of Government; University of Oxford, 2020. https://www.bsg.ox.ac.uk/research/research-projects/covid-19-government-response-tracker (viewed Aug 2021).
- 2. University of Melbourne. The Collaborative Maternity and Newborn Dashboard for the COVID‐19 pandemic; report No. 5 (period ending 31 March 2021). https://mercyperinatal.com/project/collaborative-maternity-and-newborn-dashboard-for-the-covid-19-pandemic (viewed Aug 2021).
- 3. Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust 2012; 197: 291–294. https://www.mja.com.au/journal/2012/197/5/australian-national-birthweight-percentiles-sex-and-gestational-age-1998-2007
- 4. Taylor MM, Kobeissi L, Kim C, et al. Inclusion of pregnant women in COVID‐19 treatment trials: a review and global call to action. Lancet Glob Health 2021; 9: e366‐e371. Erratum in: Lancet Glob Health 2021; 9: e366‐e371.
- 5. Whitehead CL, Walker SP. Consider pregnancy in COVID‐19 therapeutic drug and vaccine trials. Lancet 2020; 395: e92.





The CoMaND project is funded by a philanthropic grant from the Norman Beischer Medical Research Foundation and a University of Melbourne Department of Obstetrics and Gynaecology Innovation grant. The CHOPAN project is funded by a grant from Ferring COVID‐19 Investigational Grants and the University of Melbourne. The funding sources did not have any role in the planning, study design, data analysis, writing or publication of this work.
The health services participating in the CoMaND and CHOPAN projects include: Mercy Health (Mercy Hospital for Women, Werribee Mercy Hospital); the Royal Women’s Hospital, the Women’s at Sandringham; Monash Health (Monash Medical Centre, Casey Hospital, Dandenong Hospital); Northern Health (the Northern Hospital); Western Health (Joan Kirner Women’s and Children’s Hospital); Eastern Health (Box Hill Hospital, the Angliss Hospital); Peninsula Health (Frankston Hospital). We thank all the site investigators and data managers for their contribution to these projects.
No relevant disclosures.