The research response in Australia has been rapid, but better coordination is imperative
The coronavirus disease 2019 (COVID‐19) pandemic has seen clinical trials launched at exceptional speed in unprecedented numbers.1 While this is a positive development, the rapidity of trial launches and the unpredictable nature of the pandemic bring challenges for the conduct of trials and evidence synthesis. Duplication of effort is a risk, and many trials alone are underpowered to find statistically significant effects for clinically important outcomes, including mortality.1 In addition, the hard‐to‐predict waves of the pandemic may hinder recruitment due to declining cases or pose challenges to starting trials quickly in emerging hotspots.2 Recruitment has been a particular issue in Australia due to low case numbers compared with other countries. Furthermore, funds in Australia were rapidly made available to support research addressing the pandemic, but little is known about how effectively these funds have been used to drive the global agenda of preventing, diagnosing and treating COVID‐19. We aimed to derive an understanding of the current landscape of clinical trials addressing the COVID‐19 pandemic in Australia and to what extent Australian researchers have responded to the global need for coordination and collaboration. Therefore, we searched the Australian New Zealand Clinical Trials Registry (ANZCTR) and ClinicalTrials.gov from 1 January to 16 November 2020, as these sources capture approximately 95% of registered trials recruiting in Australia.3
Research scale‐up
The research scale‐up in Australia during the COVID‐19 pandemic has been impressive. Of 1637 studies registered, 1174 were interventional studies with a recruitment site in Australia. Of these, 56 were COVID‐19 trials, targeting 33 757 participants (Supporting Information, figure 1). The trials characteristics are summarised in Box 1 (detailed information is included in the Supporting Information, tables 1–3). Four trials (7%) were completed and the remainder were recruiting (n = 26, 46%), not yet recruiting (n = 24, 43%), or withdrawn (n = 2, 4%; Supporting Information, tables 1–3). Most trials (n = 46, 82%) recruited only in Australia, while ten trials (16%) recruited in Australia and internationally. Forty trials (71%) had no commercial sponsor, and were funded by government or not‐for‐profit sources. Only seven trials (12%) included populations at high risk of poor outcomes from COVID‐19 such as people with comorbidities (eg, cancer, cardiovascular disease, chronic kidney disease).
Nineteen (35%) were prevention trials. We identified ten (18%) vaccine trials, of which two repurposed existing vaccines for COVID‐19 prevention and eight investigated efficacy using a COVID‐19‐specific antigen. Thirty‐four (62%) were treatment trials, of which 22 (39%) were drug trials. A broad array of drug categories was investigated, including but not limited to immunosuppressants, immunostimulants, stem cell therapies, antivirals and anti‐inflammatories. The merits, risks and proposed solutions of included trials are summarised in Box 2.
We identified an additional 12 COVID‐19‐related trials assessing indirect effects of the pandemic (Box 1 and Supporting Information, table 4). The majority (n = 11, 92%) investigated mental health issues related to uncertainty and isolation during the pandemic.
The impact of fast track procedures on scientific rigour and research prioritisation
The other side of the rapid emergence of trials is the haste with which funding, development and implementation happened, leading to concerns about research waste and prioritisation, and ethical and scientific rigour.4 Adding concern is the fact that no full, publicly available protocols were identified for any of the included trials. Most organisations did not have fast track procedures in place at the start of the pandemic.4 The development of publicly available, transparent procedures and standards for all stages of clinical trials (eg, development, funding, ethics, conduct, dissemination) should be an important lesson from the COVID‐19 pandemic. Such standards must balance the urgency of advancing knowledge with the retention of ethical and scientific rigour.
Most of the included trials (89%) tested pharmaceutical drugs or devices, except for six trials: three telehealth applications for COVID‐19‐monitoring and rehabilitation, one lifestyle intervention, and one intervention on patient positioning during oxygenation. There were no trials on public health communication, community transmission prevention or long COVID‐19 symptoms, pointing to omissions in research prioritisation. Furthermore, extensive media coverage and public opinion may have influenced prioritisation of interventions that were not particularly promising.1,5 For instance, many simultaneous trials on hydroxychloroquine (six in Australia alone) may have put many patients unnecessarily at risk.
Untapped potential of innovative study designs
Australian COVID‐19 trials tested a range of innovative interventions, such as vaccine techniques (nanoparticles and the delivery of genetic material in two vaccine trials) and digital health solutions for home‐monitoring of mild COVID‐19. Triallists demonstrated adaptability to transmission risks for in‐person contact through innovation in trial conduct, with digital recruitment and delivery modes such as video calls or smartphone applications. Yet, innovation in trial design was lacking. We identified only two trials using adaptive methods (Bayesian designs), which can respond to the rapidly changing landscape of treatment options and thus deliver results more efficiently.
Trials often underpowered for clinical outcomes
The median target sample size was small (150; interquartile range, 33–395), meaning that, individually, trials were likely underpowered to detect differences in clinically important outcomes.6 For example, to detect a 30% relative reduction in mortality (similar to that observed for corticosteroids7) with 80% power, a sample size of 4424 in a hospitalised population (with a baseline mortality rate around 7%8) would be needed (Supporting Information, figure 2). None of the identified treatment trials are sufficiently powered to detect such a difference in mortality; and with low case numbers in Australia, it seems unlikely that a single trial could obtain such large sample sizes.
Limited collection of core outcomes precludes evidence synthesis
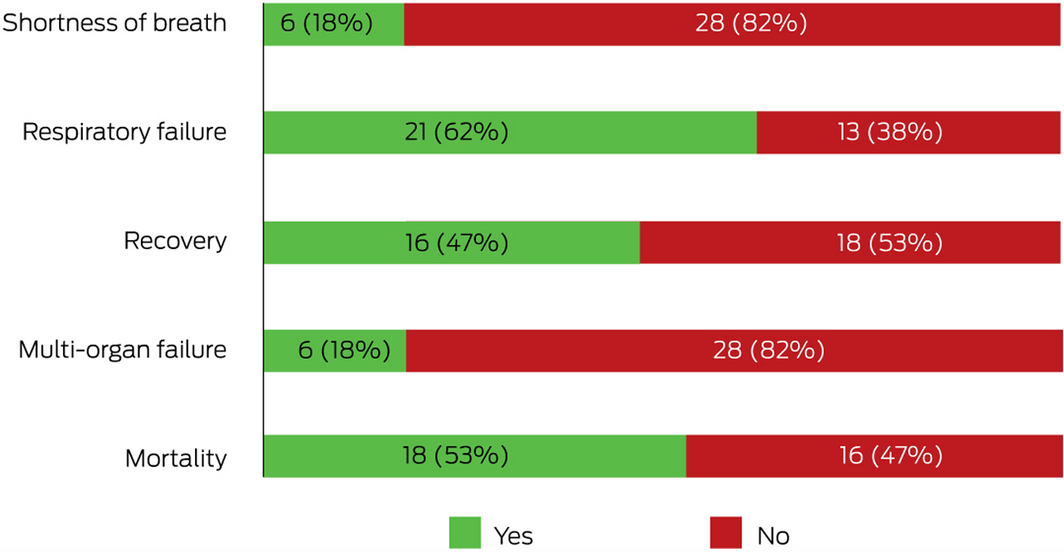
Evidence synthesis in the form of a meta‐analysis across trials is critical to obtain sufficient power to detect differences in core outcomes or subgroups of participants, particularly when individual trials are underpowered. Core outcome sets were agreed early in the pandemic and are evolving.6 We assessed availability of the identified core outcomes mortality, respiratory failure, multi‐organ failure, shortness of breath, and recovery (Supporting Information, table 5).4 Of the 34 COVID‐19 treatment trials in Australia, the proportion assessing each core outcome was low (Box 3). For instance, only 53% (18 trials) assessed mortality, and 18% (six trials) assessed shortness of breath, whereas 63% (21 trials) assessed respiratory failure. Only one trial included all core outcomes, and ten trials (29%) included none. Thus, it will be impossible to synthesise results or make important comparisons for many of the trials.
Data sharing intentions low
The International Committee of Medical Journal Editors (ICMJE) declared data sharing an ethical obligation9 to honour the risk trial participants take by increasing the likelihood that their participation results in useful findings.2,9 Since the COVID‐19 pandemic began, there have been several high profile calls for collaboration and data sharing across studies to enable more complex analyses and reliable effect estimates than would be obtained by simple combination of aggregate data.2,10 These calls seem to pass largely unheard among triallists in Australia, with 80% (41 trials) indicating they are not planning to share data (Supporting Information, table 6). While these declarations at trial outset may be conservative and investigators may decide to share data later, they are still concerning. Frequently mentioned barriers to data sharing include a lack of understanding of the relevance, lack of resources to prepare data, insufficient academic recognition, and concerns about participant privacy, ethics approval and data misuse.11 Structural support by funding bodies, research institutions, ethics committees and journal editors is needed to address barriers and facilitate data sharing.11 This could include a recognition system for collaboration and data sharing and standardised moderated processes for data sharing following FAIR (findable, accessible, interoperable, reusable) principles.12 No recognised FAIR data repository is yet available in Australia.12
Opportunities for strategic coordination and collaboration
As the COVID‐19 pandemic evolves, the clinical and societal need for research evidence will continue. There may be shifts in research focus as our understanding of COVID‐19 grows, perhaps to “long COVID‐19” or other sequelae. Coordinating research efforts is a cost‐effective, more reliable and timely way of achieving larger sample sizes and, thus, more impactful research evidence. Prospective meta‐analyses and other next generation systematic review approaches provide suitable frameworks to coordinate such collaborative research efforts and to align key elements of study design, such as core outcomes.13,14 Internationally, researchers have begun applying these frameworks to the pandemic,2,10 including an influential prospective meta‐analysis evaluating corticosteroid treatment for COVID‐19.7
In Australia, the COVID‐19 pandemic has led to rapid changes in some processes including fast‐tracked funding, ethics approvals, trial registration, and publication.15 Yet, too little has happened in creating infrastructure and funding for rapid collaboration, advanced adaptive methodologies and data sharing. In future, with adequate funding for technological innovation, clinical trial registries may play a key role in automatically connecting similar trials and facilitating collaboration. The COVID‐19 pandemic presents a unique opportunity to improve collaborative infrastructure and methodologies, and advance future research across all health areas.
Box 1 – Characteristics of coronavirus disease 2019 (COVID‐19) trials and COVID‐19‐related trials
|
Characteristics |
COVID‐19 trials |
COVID‐19‐related trials |
Overall |
||||||||||||
|
|
|||||||||||||||
|
Total number of trials |
56 |
12 |
68 |
||||||||||||
|
Total participants across trials |
33 757 |
2586 |
36 343 |
||||||||||||
|
Participants per trial |
|
|
|
||||||||||||
|
Median (IQR) |
150 (33–395) |
147 (94–280) |
150 (37–395) |
||||||||||||
|
Mean (SD) |
625 (1507) |
215 (184) |
551 (1374) |
||||||||||||
|
Trial status |
|
|
|
||||||||||||
|
Not yet recruiting |
24 (43%) |
9 (75%) |
33 (49%) |
||||||||||||
|
Recruiting |
26 (46%) |
3 (25%) |
29 (43%) |
||||||||||||
|
Completed |
4 (7%) |
0 |
4 (5.9%) |
||||||||||||
|
Withdrawn |
2 (4%) |
0 |
2 (2.9%) |
||||||||||||
|
Trial phase* |
|
|
|
||||||||||||
|
Phase 0 |
1/55 (2%) |
0 |
1 (1%) |
||||||||||||
|
Phase 1 |
19/55 (35%) |
1 (8%) |
20 (30%) |
||||||||||||
|
Phase 2 |
5/55 (9%) |
0 (0%) |
5 (7%) |
||||||||||||
|
Phase 3 |
14/55 (25%) |
0 (0%) |
14 (21%) |
||||||||||||
|
Phase 4 |
2/55 (4%) |
0 (0%) |
2 (3%) |
||||||||||||
|
Not applicable (eg, not a drug trial) |
14/55 (25%) |
11 (92%) |
25 (37%) |
||||||||||||
|
Recruitment country |
|
|
|
||||||||||||
|
Australia only |
46 (82%) |
11 (92%) |
57 (84%) |
||||||||||||
|
International (Australia and other country/countries) |
10 (18%) |
1 (8%) |
11 (16%) |
||||||||||||
|
Purpose* |
|
|
|
||||||||||||
|
Treatment, drug |
24/55 (44%) |
0 |
24 (36%) |
||||||||||||
|
Treatment, other |
10/55 (18%) |
9 (75%) |
19 (28%) |
||||||||||||
|
Prevention, vaccine† |
8/55 (14%) |
0 |
8 (12%) |
||||||||||||
|
Prevention, other |
11/55 (20%) |
2 (17%) |
13 (19%) |
||||||||||||
|
Other (eg, diagnosis, education) |
2/55 (4%) |
1 (8%) |
3 (5%) |
||||||||||||
|
Included population |
|
|
|
||||||||||||
|
Confirmed COVID‐19 |
34 (61%) |
1 (8%) |
35 (51%) |
||||||||||||
|
Healthy volunteers |
14 (25%) |
3 (25%) |
17 (25%) |
||||||||||||
|
Health care professional |
6 (11%) |
2 (17%) |
8 (12%) |
||||||||||||
|
Individuals at high risk of poor outcomes |
2 (4%) |
6 (50%) |
8 (12%) |
||||||||||||
|
Population age |
|
|
|
||||||||||||
|
Adult (18–65 years) |
9 (16%) |
1 (8%) |
10 (15%) |
||||||||||||
|
Older adult (age > 65 years) |
2 (4%) |
1 (8%) |
3 (4%) |
||||||||||||
|
All ages |
45 (80%) |
10 (83%) |
55 (81%) |
||||||||||||
|
Any blinding (personnel or participant)* |
|
|
|
||||||||||||
|
Yes |
19/49 (37%) |
3/8 (38%) |
22 (37%) |
||||||||||||
|
No |
30/49 (63%) |
5/8 (62%) |
38 (63%) |
||||||||||||
|
Randomisation* |
|
|
|
||||||||||||
|
Randomised controlled trial |
44/54 (81%) |
7 (58%) |
51 (77%) |
||||||||||||
|
Non‐randomised trial |
10/54 (19%) |
5 (42%) |
15 (23%) |
||||||||||||
|
Trials using digital health solutions |
|
|
|
||||||||||||
|
Yes |
5 (9%) |
12 (100%) |
17 (25%) |
||||||||||||
|
No |
51 (91%) |
0 |
51 (75%) |
||||||||||||
|
Commercial involvement (sponsorship, collaboration or funding) |
|
|
|
||||||||||||
|
No commercial involvement |
40 (71%) |
9 (75%) |
49 (72%) |
||||||||||||
|
Commercial involvement |
16 (29%) |
3 (25%) |
19 (28%) |
||||||||||||
|
Population with comorbidity |
|
|
|
||||||||||||
|
Yes |
7 (12%) |
2 (17%) |
9 (13%) |
||||||||||||
|
No |
49 (88%) |
10 (83%) |
58 (87%) |
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. * No information available for one trial for trial phase and purpose, for seven trials for blinding (optional registration field), and for two trials for study design for COVID‐19 trials. For COVID‐19‐related trials, no information was available for four trials for blinding (optional registration field). These trials were excluded from the analysis for these characteristics. One trial with a sample size of 30 000 was excluded from the sample size analyses. † Two vaccine clinical trials registered on the Australian New Zealand Clinical Trials Registry (ANZCTR) were recorded as “treatment” for the “purpose of the study” field. |
|||||||||||||||
Box 2 – Merits, risks and proposed solutions for coronavirus disease 2019 (COVID‐19) trials in Australia
|
|
Merits |
Risks and research gaps |
Proposed solutions |
||||||||||||
|
|
|||||||||||||||
|
Speed of response |
Rapid response to emerging pandemic |
Haste in funding, development and implementation may have jeopardised scientific and ethical rigour |
Develop protocols for fast track procedures in emergency scenarios balancing rigour and urgency |
||||||||||||
|
Number of trials and sample size |
Impressive research scale‐up, many trials being launched |
Most trials relatively small, limited statistical power to detect effects on clinically important outcomes (eg, mortality) |
Consider evidence synthesis opportunities throughout trial conduct, facilitate collaboration and coordination to enable pooling of data and results |
||||||||||||
|
Core outcomes and evidence synthesis |
Core outcomes have been developed early in the pandemic to enable successful evidence synthesis |
Data sharing/collaboration intentions are low |
Encourage and create infrastructure for collaboration (eg, in prospective meta‐analyses) through funding bodies and trial registries |
||||||||||||
|
|
|
Low proportion of trials collecting core outcomes (eg, only 53% assess mortality) |
Establish a recognition system for collaboration and data sharing following FAIR principles |
||||||||||||
|
Innovation |
Range of innovative interventions (eg, vaccine solutions and digital health solutions) balanced with repurposing of existing treatments |
Lack of innovation in trial design (eg, only two trials using adaptive designs) |
Increase use of adaptive designs to respond to the rapidly changing evidence landscape |
||||||||||||
|
|
Innovation in trial conduct (digital recruitment and delivery modes) |
|
|
||||||||||||
|
Types of interventions and populations studied |
Broad array of drug categories investigated |
Extensive media coverage and public opinion may have misled research prioritisation (eg, too many hydroxychloroquine trials) |
Improve research coordination and prioritisation through infrastructure (eg, funders, trial registries) to ensure a variety of priorities are met and to avoid duplication of effort |
||||||||||||
|
|
|
Few non‐pharmaceutical trials and no trials on public health communication or community transmission prevention |
|
||||||||||||
|
|
|
Few trials included populations at high risk of poor outcomes from COVID‐19 such as those with comorbidities |
|
||||||||||||
|
|
|||||||||||||||
|
FAIR = findable, accessible, interoperable, reusable. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust 2020; 213: 86–93. https://www.mja.com.au/journal/2020/213/2/clinical-trials-prevention-and-treatment-covid-19-current-state-play
- 2. Petkova E, Antman EM, Troxel AB. Pooling data from individual clinical trials in the COVID-19 era. JAMA 2020; 324: 543–545.
- 3. Askie L, Hunter K, Berber S, et al. The clinical trials landscape in Australia 2006–2015. Sydney: Australian New Zealand Clinical Trials Registry, 2017. https://www.anzctr.org.au/docs/ClinicalTrialsInAustralia2006-2015.pdf (viewed Apr 2021).
- 4. Bahans C, Leymarie S, Malauzat D, et al. Ethical considerations of the dynamics of clinical trials in an epidemic context: studies on COVID-19. Ethics Med Public Health 2021; 16: 100621.
- 5. Saag MS. Misguided use of hydroxychloroquine for COVID-19: the infusion of politics into science. JAMA 2020; 324: 2161–2162.
- 6. Tong A, Elliott JH, Azevedo LC, et al. Core outcomes set for trials in people with coronavirus disease 2019. J Crit Care Med 2020; 48: 1622–1635.
- 7. WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group; Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341.
- 8. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. J Crit Care Med 2021; 49: 209–214.
- 9. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Ann Intern Med 2017; 167: 63–65.
- 10. Ma Z, Liu J, Pan Q. Overwhelming COVID-19 clinical trials: call for prospective meta-analyses. Trends Pharmacol Sci 2020; 41: 501–503.
- 11. Tan AC, Askie LM, Hunter KE, et al. Data sharing — trialists’ plans at registration, attitudes, barriers and facilitators: a cohort study and cross-sectional survey. Res Synth Methods 2021; https://doi.org/10.1002/jrsm.1500 [Epub ahead of print].
- 12. International Consortium of Investigators for Fairness in Trial Data Sharing; Devereaux PJ, Guyatt G, Gerstein H, et al. Toward fairness in data sharing. N Engl J Med 2016; 375: 405–407.
- 13. Seidler AL, Hunter KE, Cheyne S, et al. Prospective meta-analyses and Cochrane’s role in embracing next-generation methodologies. Cochrane Database Syst Rev 2020; (10): ED000145.
- 14. Seidler AL, Hunter KE, Cheyne S, et al. A guide to prospective meta-analysis. BMJ 2019; 367: l5342.
- 15. Whitmore KA, Laupland KB, Vincent CM, et al. Changes in medical scientific publication associated with the COVID-19 pandemic. Med J Aust 2020; 213: 496–469. https://www.mja.com.au/journal/2020/213/11/changes-medical-scientific-publication-associated-covid-19-pandemic





We thank Melina Willson, ANZCTR manager, for her input to the protocol and earlier drafts of this perspective, and Sol Libesman, for providing technical support.
No relevant disclosures.