The known: Reflecting the uncertain evidence base, guidelines offer conflicting advice about the value of clinical breast examination for breast cancer surveillance of women with BRCA1/2 mutations.
The new: We found that the sensitivity of clinical breast examination for detecting cancers was very low. It is not useful for the surveillance of women with BRCA1/2 mutations undergoing routine MRI screening.
The implications: Clinical breast examination can safely be omitted from breast cancer screening of women with BRCA1/2 mutations. This could reduce consultation times and facilitate the use of telehealth.
In 2020, more than 19 000 women in Australia were diagnosed with breast cancer.1 The lifetime risk of breast cancer for women with mutations in the breast cancer predisposition genes BRCA1 and BRCA2 is about 70%, compared with 14% for the general population.2 These women are offered several strategies to reduce their risk, including risk‐reducing bilateral mastectomy, risk‐reducing medications, and management of lifestyle factors. Women who opt not to have risk‐reducing bilateral mastectomy are offered an intensive surveillance program with the aim of early detection of any breast cancer.3
In Australia, radiologic surveillance of women at high risk generally involves annual mammography and, for women under 50 years of age, magnetic resonance imaging (MRI).3 Clinical breast examination has not been included in Australian cancer management guidelines on the eviQ website since 2015,3 but the Royal Australian College of General Practitioners guidelines recommend an individualised screening program that can include breast examination.4
The reported performance of breast examination for detecting breast cancer is highly variable; in women at high risk, sensitivity ranges between 0 and 64.1% and specificity between 95.9% and 99.3%.5,6,7,8,9,10,11,12,13,14,15,16 Its reported sensitivity in women with BRCA1/2 mutations is 0–13%.5,6,7,8
Several factors may explain the variation in sensitivity of breast examination for detecting breast cancer. Firstly, test‐related variation (ie, the level of skill of the clinician performing the breast examination) can affect sensitivity and specificity. Secondly, the utility of breast examination is probably influenced by the results of concurrent radiologic screening; breast examination may detect fewer cancers in women undergoing regular MRI screening, as most would be detected by MRI while impalpable.7,8,9,10,11 The highest reported sensitivity for breast examination (64.1%) was associated with a screening program that did not include MRI.15
It is important to know whether adding breast examination to routine radiologic screening improves cancer detection. Breast examination can be uncomfortable for women, and requires a longer consultation and review in person rather than a telehealth appointment. We therefore estimated the sensitivity and specificity of breast examination, and the number of breast cancers detected by breast examination alone in women with BRCA1/2 mutations participating in a screening program that includes mammography for all screened women and (since the introduction of a Medicare rebate in 2009) MRI for those under 50 years of age.17 We hypothesised that few breast cancers would be detected in this setting by breast examination alone.
Methods
Women without a personal history of cancer, but with a high familial or genetic risk of breast or ovarian cancer, can attend the Breast and Ovarian Cancer Risk Management Clinic at the Peter MacCallum Cancer Centre in Melbourne at intervals of 6 to 12 months.18 We undertook a retrospective study of data for consecutive women with pathogenic BRCA1/2 gene mutations who had attended the clinic at least twice between its opening on 1 September 2001 and 31 December 2019. Women were excluded if they had undergone risk‐reducing bilateral mastectomy prior to their second clinic visit.
Data collection
All data were extracted from personal medical records and managed with REDCap 9.5 electronic data capture tools; the REDCap database is hosted at the University of Melbourne.
Breast examination includes inspection and palpation of both breasts and axillary lymph nodes. Women attending the risk management clinic undergo breast examination at intervals of 6 to 12 months by medical oncologists or breast surgeons, who may be aware of the results of concurrent imaging. For some women, their general practitioner performed breast examinations outside the clinic at alternating six‐monthly intervals; only breast examinations during routine risk management clinic visits were included in our analysis. If women presented to the clinic because of imaging evidence of an abnormality, the breast examination result was excluded from our analysis. Information on adverse events related to breast examination was not collected.
The standard screening protocol at the risk management clinic has changed over time, but since 2009 MRI has normally been performed annually from age 25–30 years (depending on family history) until age 50 years. A baseline mammogram is generally performed when a woman is 30 years old to assess breast density; if high, annual mammography is commenced at a later age because of its low sensitivity in women with dense breasts. Some women underwent ultrasound screening while pregnant or breast‐feeding. Most imaging modalities were performed at the Peter MacCallum Cancer Centre and interpreted by specialist radiologists.
A tick‐box template included in MacCallum Cancer Centre medical records is used to document the screening investigations (breast examination, mammogram, MRI) performed and their results (normal, abnormal) (Supporting Information, figure). We also extracted radiologic screening results from radiologist reports. If abnormal, any additional investigations (early interval imaging, ultrasound, biopsy) are also recorded. The reference standard for breast cancer diagnosis was histopathological confirmation of breast cancer on biopsy. Pathologists were not blinded to clinical history or screening results.
Outcomes
The primary endpoint was a breast cancer diagnosis (either ductal carcinoma in situ or invasive breast cancer). Secondary endpoints were breast examination false positive results and the stage and phenotype of breast cancers detected by breast examination.
Statistical analysis
We included data for all women who met our eligibility criteria. Participant data were censored at the earliest date for bilateral risk‐reducing mastectomy, breast cancer diagnosis, final clinic visit, or death. Patient demographic and baseline characteristics and treatment details, and data for cancers detected by breast examination, are summarised as means with standard deviations (SDs) or medians with interquartile ranges (IQRs) and ranges for continuous variables, and as numbers and proportions for categorical variables. Clinic visits with inadequate documentation of breast examination were excluded from our analysis. Indeterminate reference standard results were treated as negative results, as only positive results lead to breast cancer diagnoses. We calculated the rate of breast cancer diagnosis based on breast examination (95% confidence interval [CI]), and sensitivity, specificity, positive and negative predictive values, and false positive rates for breast examination on a per patient basis. Sensitivity of breast examination was defined as the proportion of all breast cancers diagnosed during the study period, regardless of diagnostic pathway, that were detected by breast examination alone (ie, the results of any radiologic screening were normal). Statistical analyses were performed in R 3.6.3 (R Foundation for Statistical Computing).
Ethics approval
The study was approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee (HREC 19/230R), which waived the requirement for individual participant consent. The approved study protocol is available from the corresponding author.
Results
Of the 558 women with BRCA1 and BRCA2 mutations who attended the risk management clinic during 2001‒2019, 414 met the eligibility criteria (Box 1); 186 women with BRCA1 mutations and 228 with BRCA2 mutations underwent 1761 woman‐years of follow‐up (Box 2; Supporting Information, table). The mean age at the first clinic visit was 35.5 years (SD, 11.2 years). A total of 2723 risk management clinic visits were recorded, for 2552 of which screening breast examinations were documented (94%; median number of visits per woman: five; IQR, 3–9 visits).
Breast events
Data for 98 women were censored at the time of bilateral risk‐reducing mastectomy (24%). A total of 35 women (19 with BRCA1, 16 with BRCA2 mutations) were diagnosed with breast cancer; 27 were screen‐detected, eight were interval cancers. Thirteen ductal carcinomas in situ and 20 invasive cancers were identified; details were unavailable in two cases, as treatment was undertaken at another institution (Box 3).
Seven of the eight women with interval cancers had presented with self‐detected breast lumps between clinic visits, each less than two months before their next scheduled radiologic and breast examination screenings. One of these women had given birth five months earlier, was breastfeeding, and had not resumed regular screening. The eighth woman was also deemed to have an interval cancer because her ductal carcinoma in situ (35 mm) was histopathologically confirmed at bilateral risk‐reducing mastectomy, although MRI screening one month earlier had indicated the possibility of ductal carcinoma in situ. Seven of the women with interval cancers had BRCA1 mutations, and most of the cancers were aggressive, invasive, high grade, and hormone receptor‐negative.
Performance of clinical breast examination
Twenty‐eight abnormal breast examination results were reported for 26 women, and six of these results were followed by biopsies; the median time between breast examination and biopsy was 13.5 days (IQR, 6.8–18.0 days; range, 0‒29 days). The biopsy results indicated breast cancer in three women, normal breast tissue in one, and a benign abnormality in one; one result was non‐diagnostic. Concurrent imaging did not identify features suggesting malignancy in the woman with the non‐diagnostic result or in the 20 women (22 abnormal breast examination results) without biopsies (Box 1).
In the three women diagnosed with breast cancer, one cancer was also detected by concurrent screening mammography; that is, two of 35 breast cancers were detected by breast examination alone (sensitivity, 6%; 95% CI, 1–19%). The specificity of clinical breast examination was 97% (95% CI, 95–98%), the positive predictive value 14% (95% CI, 2–43%), and the negative predictive value 92% (95% CI, 89–94%) (Box 4). The true positive rate for breast examination was 14% (2 of 14; 95% CI, 2–43%) and the false positive rate was 3.2% (12 of 367; 95% CI, 2–55%).
Women with breast cancers detected by clinical examination alone
A 36‐year‐old woman with a BRCA1 mutation was diagnosed with breast cancer in 2006 during her breast examination at the risk management clinic between two annual mammography screens. She had not undergone MRI screening, as it was not subsidised by Medicare until 2009; she was found to have clinically abnormal axillary nodes and further imaging detected a primary breast cancer. Annual MRI screening would now be recommended for a woman of this age with a BRCA1 mutation. A 65‐year‐old woman with a BRCA2 mutation was similarly diagnosed with breast cancer in 2007 during a risk management clinic visit between two annual mammography screens. Annual MRI screening would not be offered to this woman, as it is not subsidised for those over 50 years of age.
Discussion
In our study of 414 women with BRCA1/2 mutations undergoing surveillance at the Peter MacCallum Cancer Centre risk management clinic, 35 breast cancers were detected. Only two were detected by breast examination alone; in neither case was the woman undergoing routine MRI screening. The sensitivity of breast examination alone was 6% (95% CI, 1‒19%) and its specificity was 97% (95% CI, 95‒98%). While an ideal screening test would be both 100% sensitive and 100% specific, there is no consensus about minimum acceptable values.19 Given its low sensitivity, however, we conclude that breast examination alone is not an acceptable test for screening women with BRCA1/2 mutations.
Eight of the 35 breast cancers diagnosed in our study were interval cancers (23%). Their characteristics and those of the women in whom they were identified were consistent with other reports.5,7,8,9,11,12
The specificity of breast examination in our study was consistent with other reports (range, 95.9‒99.3%.8,9,12,13), as was the sensitivity of breast examination in women with BRCA1/2 mutations (range, 0 to 13%5,6,7,8). Few studies have concluded that clinical breast examination should remain part of screening programs; those that did recommend it14,15 did not include women undergoing MRI screening.
Nevertheless, some guidelines still recommend breast examination for women at high risk. Unlike imaging, it is relatively inexpensive. An English study estimated that breast examination (and any subsequent investigations) cost about $26 000 per quality‐adjusted life year.6 In the cited study, nurses performed the breast examinations, the utility of which was higher because routine MRI screening was not undertaken in the study population. As neither of these factors applies in Australia, the cost is likely to be higher here.
Although non‐invasive, breast examination can be intrusive. However, surveys have found that it causes anxiety or embarrassment in fewer than 10% of women at high risk.20,21 In fact, 94% of women believed that screening breast examinations were important for early detection, and 70% were reassured by normal results; even after being informed of its limited utility, 93% wanted screening breast examinations.21 Breast examination is not appropriate for relieving cancer‐specific anxiety in women, but it can be difficult to discontinue unnecessary but ingrained health behaviours. If breast examination remains a part of screening for some women, it is important to counsel them about its limited effectiveness, particularly when intensive radiological screening is undertaken, and about the possibility of false positive results.
Models of health care delivery are changing rapidly. Providing remote health care, especially in large countries such as Australia, is important for improving access. Patient satisfaction with telehealth consultations is generally high, as they provide greater accessibility to health care and reduce time commitment and costs.22,23 Breast examination requires in person review, and its omission from screening would allow telehealth consultations as an acceptable alternative to many breast cancer risk management visits.
Limitations
We evaluated the performance of breast examination alone in a large sample of women with mutations predisposing them to breast cancer, and our study is one of the few undertaken in women for whom routine screening generally included MRI. Nevertheless, our reliance on data retrospectively extracted from clinic notes was a limitation. However, consistent routine reporting of breast examination results using the tick‐box template for clinical documentation at each clinic visit mitigated this problem. Clinicians undertaking breast examinations were aware of recent imaging results, and this may have influenced their assessments. Despite a relatively large sample size, the number of breast cancers detected was small and the confidence intervals for our sensitivity analysis were broad. Death is the standard endpoint for breast cancer screening studies,24 but we used breast cancer diagnosis because of the limits imposed by our sample size and follow‐up times. We undertook a single centre study in a dedicated cancer clinic in which breast examination is performed by experienced breast specialists; in less specialised health care settings, the yield of breast examination would probably be even lower.
Conclusion
Our findings indicate that, for women with predisposing germline mutations, omitting clinical breast examination from screening programs that include MRI would be reasonable. If MRI cannot be offered or circumstances prevent its use (eg, in breastfeeding women), breast examination may be a worthwhile surveillance tool. The removal of breast examination from clinical practice could reduce anxiety and consultation times for screened women, and allow the choice of in person or telehealth consultations for many risk management visits.
Box 1 – Selection of women for study inclusion and their assessment pathways
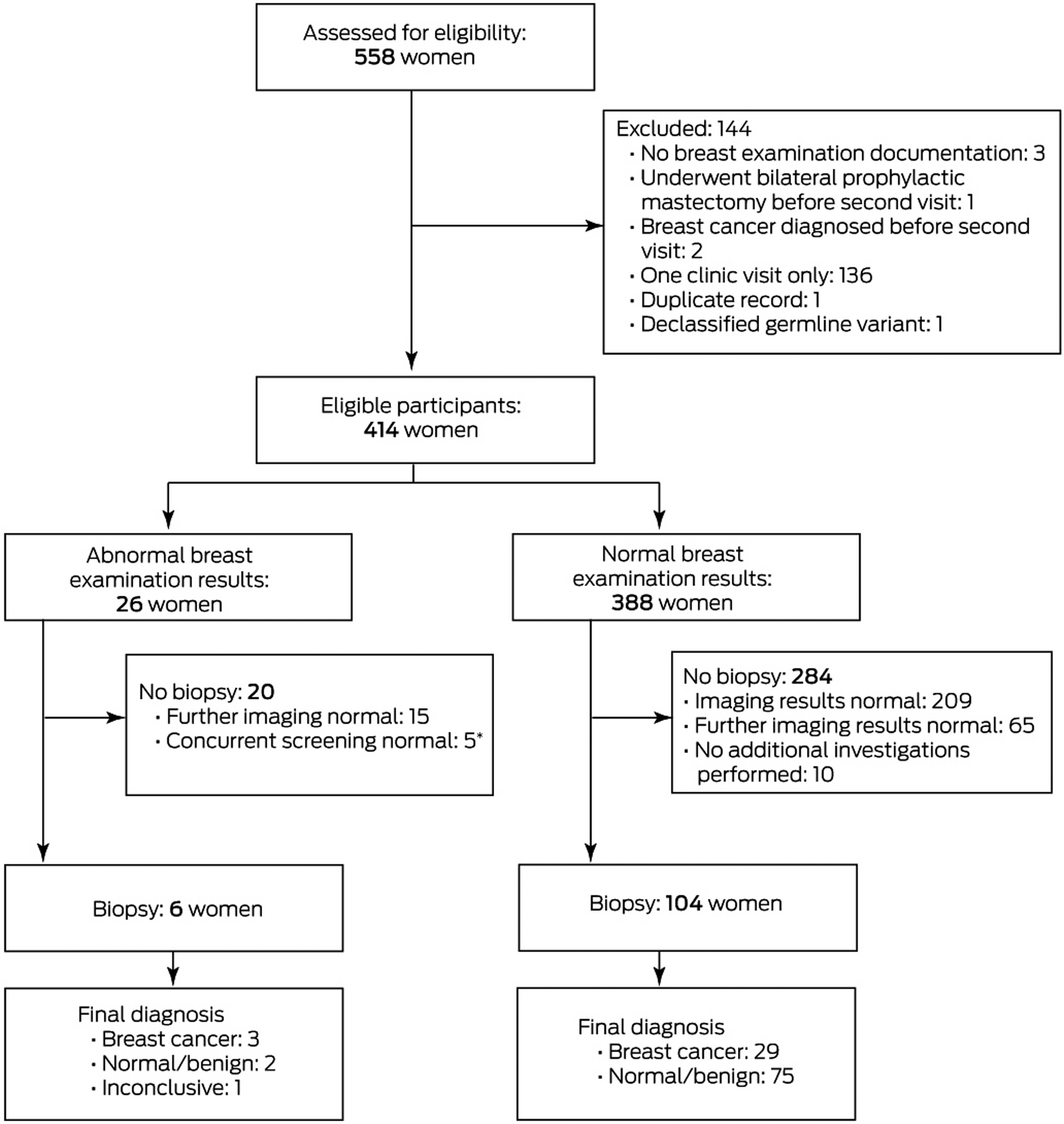
* Three women were diagnosed with breast cancer after resuming routine surveillance following abnormal breast examination results; for two of these women, the abnormal results were related to fibrocystic disease (determined by concurrent imaging). One of these two women was diagnosed with breast cancer one month later (on the basis of concurrent MRI); her abnormal breast examination result was related to fibrocystic disease in a different quadrant. The second woman was diagnosed with an interval breast cancer in the contralateral breast (self‐palpated) four months after breast examination. The third woman was diagnosed with breast cancer six years after the abnormal breast examination result; the intervening breast examinations and imaging had been normal.
Box 2 – Characteristics of the 414 women with BRCA1 and BRCA2 mutations included in the study
|
Characteristic |
Value |
||||||||||||||
|
|
|||||||||||||||
|
Age at first clinic visit (years) |
|
||||||||||||||
|
Mean (SD) |
35.5 (11.2) |
||||||||||||||
|
Range |
19.2–74.8 |
||||||||||||||
|
Length of clinical follow‐up (years) |
|
||||||||||||||
|
Median (IQR) |
3.6 (1.8–6.5) |
||||||||||||||
|
Range |
0.1–17.9 |
||||||||||||||
|
Mutation type |
|
||||||||||||||
|
BRCA1 mutation |
186 (45%) |
||||||||||||||
|
BRCA2 mutation |
228 (55%) |
||||||||||||||
|
Breast events |
|
||||||||||||||
|
Bilateral risk‐reducing mastectomy |
98 (24%) |
||||||||||||||
|
Breast cancer diagnosis |
35 (8.5%) |
||||||||||||||
|
Screen‐detected cancer |
27 |
||||||||||||||
|
Interval cancer |
8 |
||||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 3 – Characteristics of the breast cancers identified in 35 women
|
Characteristic |
Number |
||||||||||||||
|
|
|||||||||||||||
|
Mutation type |
|
||||||||||||||
|
BRCA1 |
19 |
||||||||||||||
|
BRCA2 |
16 |
||||||||||||||
|
Breast cancer type |
|
||||||||||||||
|
Ductal carcinoma in situ |
13 |
||||||||||||||
|
Invasive carcinoma, no special type |
14 |
||||||||||||||
|
Invasive carcinoma, no special type, with medullary features |
5 |
||||||||||||||
|
Invasive lobular carcinoma |
1 |
||||||||||||||
|
Missing data |
2 |
||||||||||||||
|
Estrogen receptor (ER) status |
|
||||||||||||||
|
Positive |
9 |
||||||||||||||
|
Negative |
25 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Progesterone receptor (PR) status |
|
||||||||||||||
|
Positive |
9 |
||||||||||||||
|
Negative |
25 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Human epidermal growth factor receptor 2 (HER2) status |
|
||||||||||||||
|
Positive |
3 |
||||||||||||||
|
Negative |
31 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Focality |
|
||||||||||||||
|
Multifocal |
3 |
||||||||||||||
|
Single lesion |
31 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Largest invasive breast cancer (mm) (N = 18) |
|
||||||||||||||
|
Mean (SD) |
14.3 (7.0) |
||||||||||||||
|
Median (IQR) |
15 (8.0‒22) |
||||||||||||||
|
Range |
5.0–25 |
||||||||||||||
|
Size of breast cancer (mm) |
|
||||||||||||||
|
< 20 |
22 |
||||||||||||||
|
20–50 |
11 |
||||||||||||||
|
Missing data |
2 |
||||||||||||||
|
Axillary node involvement |
|
||||||||||||||
|
Yes |
4 |
||||||||||||||
|
1–3 |
3 |
||||||||||||||
|
4–9 |
1 |
||||||||||||||
|
No |
23 |
||||||||||||||
|
Unknown (axillary surgery not performed) |
7 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
Metastatic disease present |
|
||||||||||||||
|
No |
34 |
||||||||||||||
|
Missing data |
1 |
||||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. |
|||||||||||||||
Box 4 – Performance of clinical breast examination for the 414 women included in the study
|
|
Breast cancer diagnosis |
|
|||||||||||||
|
Breast examination result abnormal, imaging results normal |
Yes |
No |
|
||||||||||||
|
|
|||||||||||||||
|
Yes |
2* |
12 |
Positive predictive value: 14% |
||||||||||||
|
No |
33 |
367 |
Negative predictive value: 92% |
||||||||||||
|
|
Sensitivity: 6% |
Specificity: 97% |
|
||||||||||||
|
|
|||||||||||||||
|
* Results of imaging tests at follow‐up visit were normal. |
|||||||||||||||
Received 14 December 2020, accepted 7 May 2021
- Tamara Hettipathirana1,2
- Courtney Macdonald2
- Jing Xie2
- Kate Moodie2
- Chris Michael2
- Kelly‐Anne Phillips1,2
- 1 University of Melbourne, Melbourne, VIC
- 2 Peter MacCallum Cancer Centre, Melbourne, VIC
Kelly‐Anne Phillips is a National Health and Medical Research Council of Australia Leadership Fellow. We thank the women whose data were analysed in this study. We also thank Michael Henderson and Paul James for their critical review of an earlier version of this manuscript.
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Cancer data in Australia (AIHW cat. no. CAN 122). Updated 13 Nov 2020. https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia (viewed Dec 2020).
- 2. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317: 2402–2416.
- 3. Cancer Institute NSW. BRCA1 or BRCA2: risk management (female) (ID 3814 v.1). eviQ Cancer Treatments Online; 2 July 2020. https://www.eviq.org.au/cancer‐genetics/adult/risk‐management/3814‐brca1‐or‐brca2‐risk‐management‐female (viewed Mar 2021).
- 4. Royal College of Australian of General Practitioners. Breast cancer. In: Guidelines for preventive activities in general practice. Ninth edition. Victoria: RACGP, 2016; pp. 109–112. https://www.racgp.org.au/clinical‐resources/clinical‐guidelines/key‐racgp‐guidelines/view‐all‐racgp‐guidelines/guidelines‐for‐preventive‐activities‐in‐general‐pr/early‐detection‐of‐cancers/breast‐cancer (viewed Apr 2021).
- 5. Rijnsburger AJ, Obdeijn IM, Kaas R, et al. BRCA1‐associated breast cancers present differently from BRCA2‐associated and familial cases: long‐term follow‐up of the Dutch MRISC Screening Study. J Clin Oncol 2010; 28: 5265–5273.
- 6. Maurice A, Evans DG, Affen J, et al. Surveillance of women at increased risk of breast cancer using mammography and clinical breast examination: further evidence of benefit. Int J Cancer 2012; 131: 417–425.
- 7. Fakkert IE, Jansen L, Meijer K, et al. Breast cancer screening in BRCA1 and BRCA2 mutation carriers after risk reducing salpingo‐oophorectomy. Breast Cancer Res Treat 2011; 129: 157–164.
- 8. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004; 292: 1317–1325.
- 9. Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010; 28: 1450–1457.
- 10. Mihalco S, Keeling S, Murphy S, O’Keeffe S. Comparison of the utility of clinical breast examination and MRI in the surveillance of women with a high risk of breast cancer. Clin Radiol 2020; 75: 194–199.
- 11. Yu J, Park A, Morris E, et al. MRI screening in a clinic population with a family history of breast cancer. Ann Surg Oncol 2008; 15: 452–461.
- 12. Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast‐enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 study): final results. Invest Radiol 2011; 46: 94–105.
- 13. Trop I, Lalonde L, Mayrand MH, et al. Multimodality breast cancer screening in women with a familial or genetic predisposition. Curr Oncol 2010; 17: 28–36.
- 14. Liljegren A, von Wachenfeldt A, Azavedo E, et al. Prospective blinded surveillance screening of Swedish women with increased hereditary risk of breast cancer. Breast Cancer Res Treat 2018; 168: 655–666.
- 15. Bennett IC, Muller J, Cockburn L, et al. Outcomes of multimodality breast screening for women at increased risk of familial breast cancer. World J Surg 2010; 34: 979–986.
- 16. Brekelmans C, Seynaeve C, Bartels C, et al. Rotterdam Committee for Medical and Genetic Counseling. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001; 19: 924–930.
- 17. Australian Department of Health. MRI (magnetic resonance imaging) breast services Q & A (questions and answers). Updated 12 Nov 2013. https://www1.health.gov.au/internet/main/publishing.nsf/Content/mri‐breast‐services‐q‐and‐a (viewed June 2020).
- 18. Antill Y, Shanahan M, Phillips K. The integrated, multidisciplinary clinic: a new model for the ongoing management of women at high genetic risk for breast and ovarian cancer. Cancer Forum 2005; 29: 107–110.
- 19. Talley N, Frankum B, Currow D. Evidence based medicine and critical appraisal of the literature. In: Essentials of internal medicine. Third edition. Sydney: Churchill Livingstone/Elsevier, 2015; pp. 5–15.
- 20. Antill YC, Reynolds J, Young MA, et al. Screening behavior in women at increased familial risk for breast cancer. Fam Cancer 2006; 5: 359–368.
- 21. Spiegel TN, Hill KA, Warner E. The attitudes of women with BRCA1 and BRCA2 mutations toward clinical breast examinations and breast self‐examinations. J Womens Health (Larchmt) 2009; 18: 1019–1024.
- 22. Orlando JF, Beard M, Kumar S. Systematic review of patient and caregivers’ satisfaction with telehealth videoconferencing as a mode of service delivery in managing patients’ health. PLoS One 2019; 14: e0221848.
- 23. Donelan K, Barreto EA, Sossong S, et al. Patient and clinician experiences with telehealth for patient follow‐up care. Am J Manag Care 2019; 25: 40–44.
- 24. Jatoi I, Pinsky PF. Breast cancer screening trials: endpoints and overdiagnosis. J Natl Cancer Inst 2020; 113: djaa140.





Abstract
Objective: To assess the sensitivity and specificity of clinical breast examination for detecting breast cancer in asymptomatic women with predisposing germline mutations enrolled in a cancer risk management program that includes radiologic screening.
Design, setting: Retrospective, longitudinal cohort study of women with BRCA1/2 mutations who attended the Breast and Ovarian Cancer Risk Management Clinic at the Peter MacCallum Cancer Centre, a tertiary referral centre in Melbourne, during 1 September 2001 – 31 December 2019.
Participants: Consecutive women with BRCA1/2 mutations who did not have personal histories of cancer and had not undergone bilateral risk‐reducing mastectomy, and who had visited the clinic at least twice during the study period. Participants had generally undergone breast examination at 6‐ or 12‐month intervals, and annual breast imaging (mammography; and magnetic resonance imaging [MRI] for women aged 50 years or younger).
Main outcome measures: Sensitivity (proportion of all biopsy‐confirmed breast cancers detected by breast examination alone) and specificity of breast examination for detecting breast cancer.
Results: Of 414 eligible women (mean age, 35.5 years; SD, 11.2 years), 35 were diagnosed with breast cancer during 1761 woman‐years of follow‐up. Only two were diagnosed based on breast examination alone (ie, without radiologic evidence), neither of whom was undergoing MRI screening. The sensitivity of breast examination was 6% (95% CI, 1‒19%), the specificity 97% (95% CI, 95‒98%); the positive predictive value was 14% (95% CI, 2‒43%), the negative predictive value 92% (95% CI, 89‒94%).
Conclusion: Clinical breast examination did not increase the number of breast cancers detected in MRI‐screened women with BRCA1/2 mutations. Removing breast examination from surveillance programs that include MRI may be reasonable for these women.