The Royal Commission into Aged Care Quality and Safety has highlighted the high rates of polypharmacy and potential medication‐related harm in residential aged care facilities (RACFs) in Australia.1 Residential medication management review (RMMR) is a government‐funded service for facilitating quality use of medicines in RACFs.2 Previous studies have found that RMMRs by accredited pharmacists and general practitioners identify a mean of 2.7–3.9 medication‐related problems per resident, and 45–84% of pharmacists’ recommendations were accepted by GPs.3 Guidelines recommend that residents should generally receive an RMMR on entering an RACF and when their clinical circumstances change,4 but annual claims data5,6 and recent research indicate that not all residents receive RMMRs.7
We examined time to first RMMR after RACF entry by analysing data for the national historical cohort of the Registry of Senior Australians (ROSA).7 In ROSA, de‐identified data collected during aged care eligibility assessments are linked to information about government‐subsidised aged care services, general practice and allied health services subsidised under the Medicare Benefits Schedule (MBS), medicines subsidised under the Pharmaceutical Benefits Scheme (PBS), and the Australian Institute of Health and Welfare National Death Index.8 Non‐Indigenous people aged 65 years or more who first entered permanent residential care during 1 January 2012 – 31 December 2015, had received an entry‐into‐care assessment within 100 days, and had received at least one PBS‐subsidised medication during the preceding year were included. Recipients of Department of Veterans’ Affairs‐funded services and people who had previously undergone RMMRs (eg, during transition care) were excluded. The cumulative incidence function was used to determine time to first MBS claim lodged by GPs for RMMRs (item code 903) or Home Medicines Reviews (HMRs) (item code 900) after entry to permanent residential care, adjusted for competing events (death, or permanent departure from the first RACF for another reason) using the Fine–Gray method,9 with follow‐up to 31 December 2016. Statistical analyses were undertaken in SAS 9.4. The University of South Australia (reference, 200489) and Australian Institute of Health and Welfare (reference, E02018/1/418) Human Research Ethics Committees provided ethics approval for the study.
A total of 176 390 residents in 2799 RACFs were followed for a median 479 days (interquartile range [IQR], 149–858 days). Median age at entry was 84 years (IQR, 79–88 years), 108 908 were women (61.7%), and 84 864 were living with dementia (48.1%). In the year preceding entry, residents received a median of 11 unique prescription medications (IQR, 8–16 medications); 109 765 (62.2%) had received at least one high risk medication (as defined by the United States Institute for Safe Medication Practices10), and 7912 (4.5%) had received HMRs in the 12 months prior to RACF entry.
By three months after RACF entry, 19.1% of residents (Wald 95% confidence interval [CI], 18.9–19.3%) had received RMMRs, 11.8% (95% CI, 11.6–11.9%) had died without RMMRs, and 5.7% (95% CI, 5.6–5.8%) had left their RACF for other reasons without RMMRs. At 12 months, 43.1% (95% CI, 42.8–43.3%) had received RMMRs, 20.6% (95% CI, 20.5–20.8%) had died without RMMRs, and 9.0% (95% CI, 8.8–9.1%) had left without receiving RMMRs. By 24 months, 49.7% (95% CI, 49.5–50.0%) had received RMMRs, 25.8% (95% CI, 25.6–26.0%) had died without RMMRs, and 10.2% (95% CI, 10.1–10.4%) had left their first RACF for other reasons without receiving RMMRs (Box).
The high burden of medication use at the time of RACF entry suggests that most residents could have benefited from RMMRs, but MBS claims for RMMRs were lodged for fewer than one in five residents within three months of RACF entry, and fewer than one in two within two years.
Our findings are generalisable to all older Australians entering RACFs, as ROSA captures data for all people aged 65 years or more who access government‐subsidised permanent residential aged care in Australia. We could not determine why residents were not referred for RMMRs, nor the impact of recent program changes2 on RMMR uptake and resident outcomes. In 2014–15, fewer GP medication review claims were reimbursed under the MBS (54 803 RMMRs, 63 872 HMRs) than pharmacist claims (93 517 RMMRs, 72 607 HMRs).5,6 Analysing GP claims may underestimate the number of RMMR reports prepared by pharmacists because GP claims are submitted after the medication management plan is discussed with the resident or family, while pharmacist claims are submitted after the report is sent to the GP.7 MBS claims may not be lodged if the full RMMR process cannot be completed (eg, because the resident died, their clinical circumstances had changed, or the RMMR report was not received or followed up), or claiming may be overlooked. Linkage with pharmacist claims data at the individual resident level could facilitate investigation of these limitations.
Despite RMMRs being a key means for minimising medication‐related harm, MBS claims for RMMRs are lodged for only a fraction of residents who enter RACFs. The potential underuse of the program may be a missed opportunity for identifying and resolving medication‐related problems in Australian RACFs.
Box – Stacked cumulative incidence function for time to first residential medication management review (RMMR), for first two years of permanent residential care*
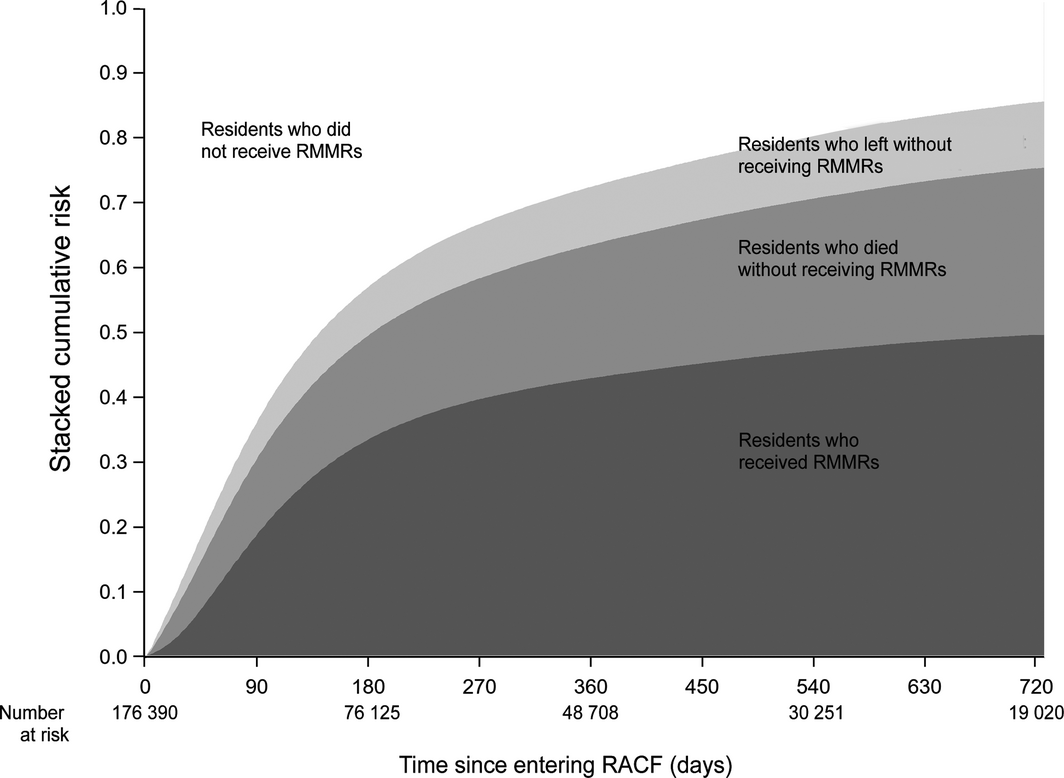
RACF = residential aged care facility. * For 176 390 residents (in 2799 residential aged facilities) included in the Registry of Senior Australians.8
Received 3 April 2020, accepted 17 August 2020
- 1. Royal Commission into Aged Care Quality and Safety. Restrictive practices. In: Interim report: Neglect, volume 1. Canberra: Commonwealth of Australia, 2019; pp. 193–216. https://agedcare.royalcommission.gov.au/publications/Documents/interim-report/interim-report-volume-1.pdf (viewed July 2020).
- 2. Pharmacy Programs Administrator. Residential medication management review and quality use of medicines. Updated July 2020. https://www.ppaonline.com.au/programs/medication-management-programs/residential-medication-management-review-and-quality-use-of-medicines (viewed July 2020).
- 3. Chen EYH, Wang KN, Sluggett JK, et al. Process, impact and outcomes of medication review in Australian residential aged care facilities: a systematic review. Australas J Ageing 2019; 38(Suppl. 2): 9–25.
- 4. The Royal Australian College of General Practitioners. RACGP aged care clinical guide (Silver Book). Revised July 2020. https://www.racgp.org.au/silverbook (viewed July 2020).
- 5. Australian Department of Health. Pharmacy programs data. https://www1.health.gov.au/internet/main/publishing.nsf/Content/pharmacy-programs-data (viewed Dec 2020).
- 6. Services Australia. Medicare item reports. http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp (viewed Dec 2020).
- 7. Sluggett JK, Bell JS, Lang C, et al. Variation in provision of collaborative medication reviews on entry to long‐term care facilities. J Am Med Dir Assoc 2021; 22: 148–155.e1.
- 8. Harrison SL, Sluggett JK, Lang C, et al. The dispensing of psychotropic medicines to older people before and after they enter residential aged care. Med J Aust 2020; 212: 309–313. https://www.mja.com.au/journal/2020/212/7/dispensing-psychotropic-medicines-older-people-and-after-they-enter-residential
- 9. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
- 10. Institute for Safe Medication Practices. ISMP list of high‐alert medications in long‐term care (LTC) settings. Nov 2017. https://www.ismp.org/sites/default/files/attachments/2017-11/LTC-High-Alert-List.pdf (viewed July 2020).





Janet Sluggett is supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship. Simon Bell is supported by an NHMRC Boosting Dementia Research Fellowship. Maria Inacio is supported by a Mid‐Career Fellowship from The Hospital Research Foundation.
Craig Whitehead is a non‐executive director of Helping Hand Aged Care. Megan Corlis was employed by Helping Hand Aged Care at the time of the study. Janet Sluggett is a registered pharmacist and accredited to perform residential medication management reviews. Janet Sluggett and Simon Bell have received grant funding from Resthaven Incorporated during the past 36 months. Janet Sluggett and Maria Inacio have received grant funding from the Australian Association of Consultant Pharmacy in the past 36 months.