The spectrum of recovery for people infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) remains uncertain.1,2,3,4 The ADAPT study is a prospective cohort study that follows up all adults diagnosed with coronavirus disease 2019 (COVID‐19) at St Vincent’s Hospital, Sydney. Our goal is to characterise the effects of infection during the 12 months after diagnosis, by initial severity of COVID‐19. Our specific aims were to determine the prevalence and nature of persistent symptoms; to evaluate lung function, health‐related quality of life, neurocognitive and olfactory abnormalities during the recovery period; and to characterise the longitudinal immune response to infection.
In this article, we report the results of assessments performed up to four months after diagnosis. All adults with SARS‐CoV‐2 infections confirmed by polymerase chain reaction (PCR) at St Vincent’s Hospital testing clinics and who could be contacted were invited to participate. Participants were prospectively assessed according to a pre‐defined schedule. The study was approved by the St Vincent’s Hospital Human Research Ethics Committee (reference, 2020/ETH00964); baseline visits commenced as soon as this approval was obtained.
Between 14 May and 21 July 2020, 78 of 167 eligible patients were enrolled (47%), with diagnosis dates between 11 March and 21 April 2020. Sixty‐nine patients had been managed in the community (30 mild, 39 moderate cases; Supporting Information, figure 1, table 1) and nine in hospital (two admitted to intensive care with acute respiratory distress syndrome). Their mean age was 47 years (standard deviation, 16 years); 27 were women, 65 had European ethnic backgrounds, and 39 had infections acquired overseas (Supporting Information, table 2). The most frequently reported comorbid conditions were hypertension (14 patients) and asthma (nine); 37 patients had no comorbidity. The most frequently reported initial COVID‐19 symptoms were fatigue (62 patients), cough (50), and headache (44). At a median of 69 days after diagnosis (interquartile range [IQR], 64–83 days), 31 patients (seven hospitalised, 24 community‐managed) had persistent symptoms, including fatigue (17 patients), shortness of breath (15), and chest tightness (14) (Box 1).
Sixty‐five patients underwent complex lung function testing at a median of 113 days (IQR, 105–131 days) after diagnosis. In eight patients (12%), total lung capacity was abnormal (ie, below lower limit of normal); median total lung capacity was significantly lower for all hospitalised patients (91% predicted; IQR, 78–99% predicted) than for community‐treated patients (102% predicted; IQR, 92–107% predicted; P = 0.023). Diffusion capacity for carbon monoxide was below the lower limit of normal in 11 patients (14%), and the median value was slightly lower for hospitalised patients (Box 2). The combination of near normal ventilatory capacity with reduced carbon monoxide diffusion capacity may indicate pulmonary vascular disease.
Neurocognitive and olfactory function were tested by trained examiners with the computerised CogState Cognitive Test Battery (cogstate.com) and the NIH Toolbox Odor Identification test.5 Cognitive impairment was evident in eight patients, in four of whom olfaction was also impaired. Five patients had mild and three had moderate cognitive impairment; psychomotor speed was the most frequent impairment. Impaired olfaction was evident in 18 patients, including four with severe anosmia. The Depression in the Medically Ill questionnaire (DMI‐10)6 was administered to 76 patients; 16 (21%) reported symptoms consistent with depression.
Considerable numbers of patients had persistent symptoms two months after SARS‐CoV‐2 infections, including fatigue, chest pain, and breathlessness. Although more frequent following severe illness, persistent symptoms were reported by 24 of 69 community‐managed patients (35%) several months after infection.
The generalisability of the findings of our single‐site study may be limited, its sample largely drawn from the highly educated, predominantly white eastern suburbs of Sydney with low rates of chronic comorbidity. Further follow‐up will provide data on the longer term trajectory of recovery after COVID‐19 and provide insights into the mechanisms of systemic inflammation after SARS‐CoV‐2 infection and its immunological correlates.
Box 1 – Symptoms of 78 patients at time of first diagnosis with SARS‐CoV‐2 infections and at first follow‐up (median, 69 days; IQR, 64–83 days)
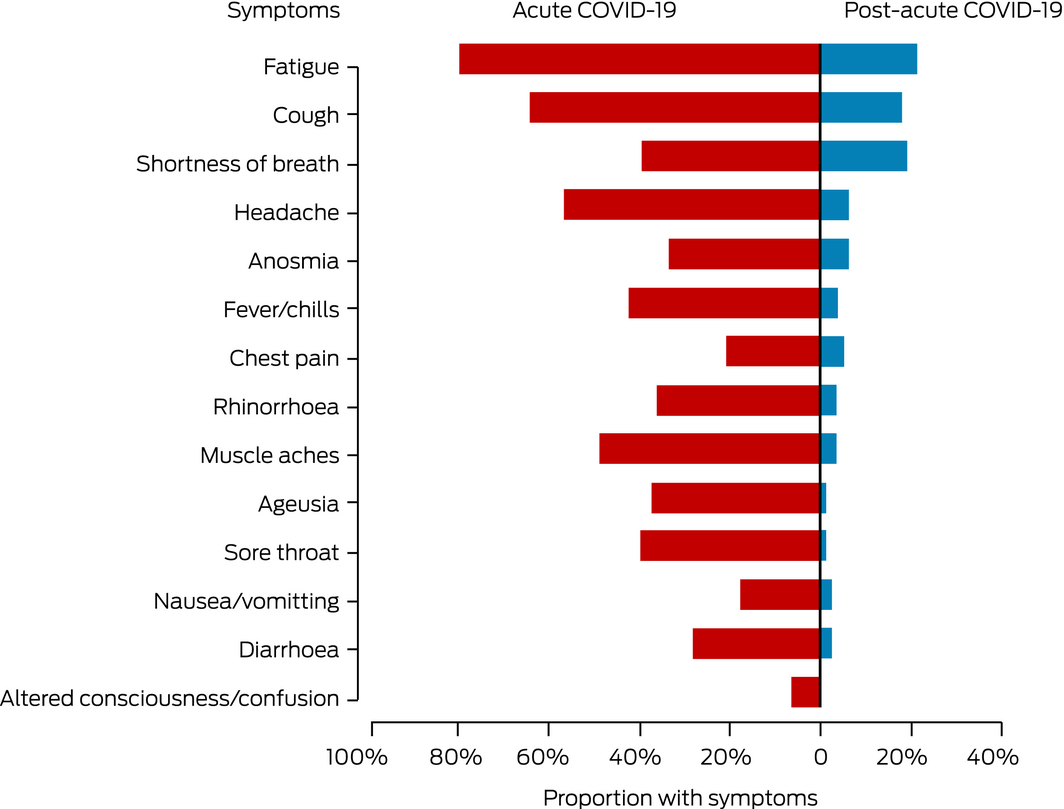
IQR = interquartile range.
Box 2 – Complex lung function testing of 65 people with SARS‐CoV‐2 infections, median of 113 days (IQR, 105–131 days) after diagnosis
Parameter |
All patients |
Community‐managed |
Hospitalised | ||||||||||||
Number of participants |
65 |
56 |
9 |
||||||||||||
Time from COVID‐19 diagnosis to first lung function (days), median (IQR) |
113 (105–131) |
115 (107–139) |
74 (63–98) |
||||||||||||
Pre‐bronchodilator FEV1 (% predicted7), median (IQR) |
99 (84–104) |
99 (84–105) |
97 (78–103) |
||||||||||||
FEV1 < lower limit of normal |
5 |
4 |
1 |
||||||||||||
Pre‐bronchodilator FVC (% predicted7), median (IQR) |
106 (97–117) |
105 (97–117) |
106 (89–118) |
||||||||||||
FVC < lower limit of normal |
3 |
2 |
1 |
||||||||||||
FEV1/FVC, mean (SD) |
74 (9.0) |
74 (8.7) |
71 (11) |
||||||||||||
Total lung capacity (% predicted8), median (IQR) |
101 (91–107) |
102 (92–107) |
91 (78–99) |
||||||||||||
Total lung capacity < lower limit of normal |
8 |
6 |
2 |
||||||||||||
DLCOcor (% predicted9), median (IQR) |
92 (84–103) |
93 (85–103) |
89 (62–99) |
||||||||||||
DLCOcor < lower limit of normal |
11 |
8 |
3 |
||||||||||||
Capillary Po2 (mmHg), mean (SD) |
86 (9.6) |
87 (9.5) |
82 (9.8) |
||||||||||||
Alveolar–arterial gradient (mmHg), mean (SD) |
24 (9.6) |
23 (9.3) |
29 (10) |
||||||||||||
COVID‐19 = coronavirus disease 2019; DLCOcor = diffusing capacity of the lung for carbon monoxide, adjusted for haemoglobin concentration; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; IQR = interquartile range; Po2 = partial pressure of oxygen; SD = standard deviation. | |||||||||||||||
Received 23 November 2020, accepted 24 December 2020
- 1. Carfì A, Bernabei R, Landi F; Gemelli Against COVID‐19 Post‐Acute Care Study Group. Persistent symptoms in patients after acute COVID‐19. JAMA 2020; 324: 603–605.
- 2. Tenforde MW, Kim SS, Lindsell CJ, et al; IVY Network Investigators; CDC COVID‐19 Response Team. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID‐19 in a multistate health care systems network; United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 993–998.
- 3. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J 2020; 55: 2001217.
- 4. Fumagalli A, Misuraca C, Bianchi A, et al. Pulmonary function in patients surviving to COVID‐19 pneumonia. Infection 2020; 28: 1–5.
- 5. Dalton P, Doty RL, Murphy C, et al. Olfactory assessment using the NIH Toolbox. Neurology 2013; 80 (11 Suppl 3): S32–S36.
- 6. Parker G, Hilton T, Bains J, Hadzi‐Pavlovic D. Cognitive‐based measures screening for depression in the medically ill: the DMI‐10 and the DMI‐18. Acta Psychiatr Scand 2002; 105: 419–426.
- 7. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343.
- 8. Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official statement of the European Respiratory Society. Eur Respir J 1995; 8: 492–506.
- 9. Stanojevic S, Graham BL, Cooper BG, et al; Global Lung Function Initiative Tlco working group. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017; 50: 1700010.





We thank the research staff at the St Vincent’s Institute for Applied Medical Research and St Vincent’s Hospital Pulmonary Function Laboratory. We appreciate grant support from the St Vincent’s Clinic Foundation and the Curran Foundation.
No relevant disclosures.