The known: Patients without coronary ischaemia attending chest pain clinics have a substantial burden of modifiable cardiovascular risk factors that is rarely explicitly discussed during the consultation. Absolute risk scores may be useful for educating patients and encouraging engagement with strategies for improving cardiovascular health.
The new: The addition of an absolute cardiovascular risk‐based, pro‐active risk factor management strategy to usual care resulted in significantly improved 5‐year risk scores at follow‐up (at least 12 months after baseline assessment).
The implications: Using absolute risk scores to promote engagement with preventive measures could play an important role in improving the cardiovascular risk profiles of patients.
The Royal Hobart Hospital established a rapid access chest pain clinic (RACPC) in 2014. It was expected1 and later confirmed2 that cardiac pathology would be identified in only about 10% of patients attending the clinic. Nevertheless, many people with chest pain are referred to the RACPC because of significant modifiable risk factors for future adverse cardiovascular events.2 One of the aims of the clinic was therefore to opportunistically treat underlying risk, even when active cardiovascular disease was excluded by clinical assessment.
Guidelines for preventing cardiovascular disease recommend various risk factor tools for estimating the risk and guiding the personalised primary prevention strategy for individual patients.3,4,5,6 In Australia, the absolute risk calculator of the Australian National Vascular Disease Prevention Alliance (NVDPA) is recommended (www.cvdcheck.org.au); it is based on the Framingham risk equation,7,8 derived from a cohort with an upper age limit of 74 years.9 This calculator cannot be used for patients with established cardiovascular disease, nor for those in groups with clinically determined high risk of cardiovascular disease, including people over 60 years of age with diabetes, and people with moderate to severe chronic kidney disease, systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg, total serum cholesterol ≥ 7.5 mmol/L, or a family history of hypercholesterolaemia.9 The NVDPA recommends tailoring risk factor management to the patient’s absolute cardiovascular risk. Lifestyle advice is recommended at all risk levels, with pharmacotherapy reserved for people at high risk (5‐year risk greater than 15%) and for those at intermediate risk (10–15%) who do not achieve risk factor reduction targets through lifestyle modification.
The absolute risk‐based approach recognises the synergism of risk factors7,10,11 and the greater overall benefit of directing preventive measures to patients at greater risk.12 Risk score calculation is designed to assist clinical decision making, but communicating risk scores to patients may also help improve risk perception and promote engagement with strategies for reducing risk.13 Few studies have evaluated the benefit of incorporating the absolute risk approach into clinical practice for these purposes,14 prompting calls to develop and test novel strategies for integrating discussions of absolute cardiovascular risk into patient–physician consultations.15
We sought to determine whether using the Australian absolute risk calculator to engage patients in pro‐active risk factor management reduced risk to a greater degree than usual care. We assessed patients in whom active cardiac pathology had been excluded after they had presented for assessment at our chest pain clinic, essentially a primary prevention population.
Methods
We undertook a prospective, randomised, open label, blinded endpoint study in the Royal Hobart Hospital RACPC to compare the benefits of an absolute risk‐guided pro‐active risk factor management strategy with those of best practice usual care. The trial was retrospectively registered with the Australia New Zealand Clinical Trial Registry (ACTRN12617000615381; 28 July 2017).
Participants
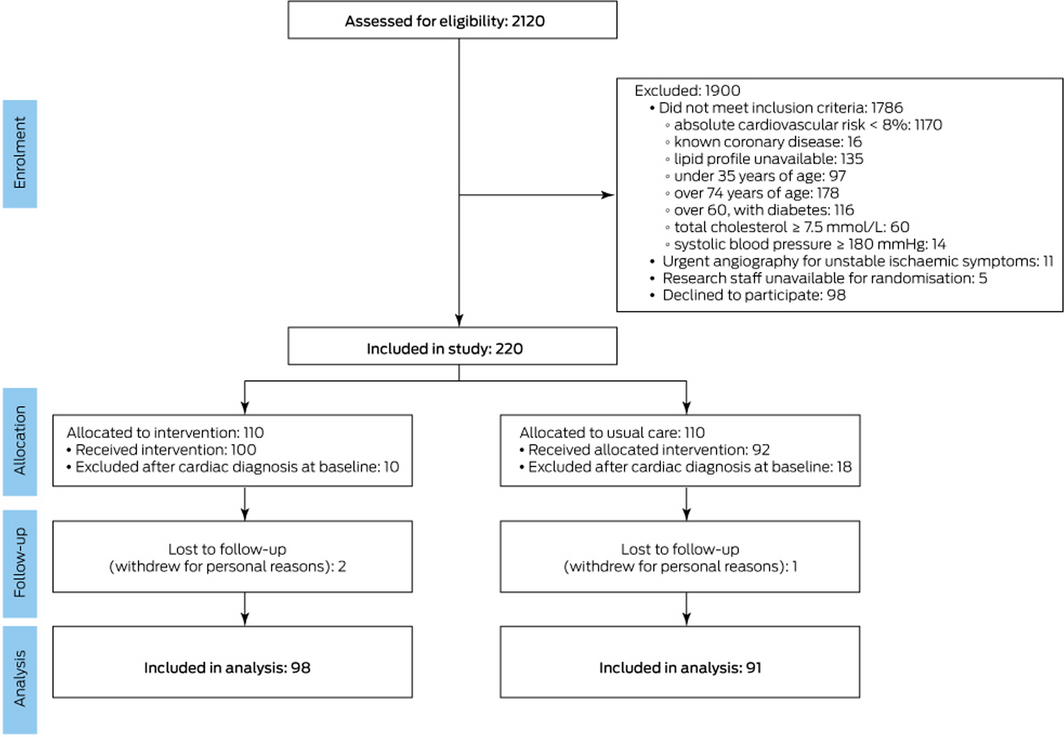
Patients who presented to the RACPC between 1 July 2014 and 31 December 2017 were screened by assessing their cardiovascular risk factors and estimating their 5‐year risk with the Australian absolute risk calculator (www.cvdcheck.org.au). Inclusion criteria were age 35–74 years and estimated 5‐year cardiovascular risk of at least 8% (to maximise the recruitment of patients for whom a reduction in cardiovascular risk would be clinically significant); exclusion criteria were known cardiac disease and pregnancy. Patients from groups with clinically determined high risk of cardiovascular disease9 were also excluded, as they should be routinely receiving aggressive management. The intervention commenced with the initial review of patients, before they were investigated for ischaemia; enrolled participants were later excluded if the baseline clinical assessment identified cardiac causes for their symptoms, as these patients were deemed candidates for secondary, rather than primary, prevention.
Protocol
Participants were randomised 1:1 (using computer‐supported block randomisation) to best practice chest pain clinic assessment (usual care) or usual care together with an absolute risk‐guided cardiovascular risk factor management strategy (intervention).
The chest pain of patients allocated to usual care was assessed by a consultant physician, cardiologist, or advanced trainee. Absolute risk scores were not discussed. Individual risk factors were discussed at the discretion of the treating clinician, consistent with standard practice in a general cardiology outpatient clinic (eg, recommending smoking cessation, or suggesting general practitioner follow‐up if blood pressure or lipid levels were significantly elevated).
In addition to chest pain assessment, patients allocated to the intervention group were counselled by the treating doctor about their 5‐year cardiovascular risk score. Individual risk factors were discussed in this context, and a strategy developed to reduce the risk score, with recommendations guided by primary prevention guidelines.9 If pharmacotherapy was indicated, it was prescribed in the clinic. Smokers were offered referral to a public smoking cessation service. The patients were provided with lifestyle advice by a registered nurse with cardiac rehabilitation experience. Participants were strongly encouraged to discuss risk management strategies with their general practitioners.
Follow‐up data collection
Research nurses, blinded to participant allocation, undertook patient reviews 12 months after the completion of randomisation for the study; that is, at least 12 months after baseline assessment. If participants were unable to attend the clinic for follow‐up, outcomes were determined on the basis of cardiovascular risk factor assessments in primary care. Adverse clinical events were determined by a review of the participants’ digital medical record by clinic staff at the time of risk factor profile follow‐up.
Outcomes
The primary outcome was change in 5‐year absolute cardiovascular risk score at follow‐up. Secondary outcomes were changes in lipid profile (total, low‐density lipoprotein [LDL] and high‐density lipoprotein [HDL] cholesterol), blood pressure, smoking status, body mass index (BMI), and major adverse cardiovascular events (composite of cardiovascular death, myocardial infarction, and stroke). Quality of life was evaluated as a health state utility value at baseline and follow‐up with the SF‐36 questionnaire and the SF‐6D multi‐attribute utility instrument Australian value set.16 The smallest clinically important difference for the SF‐6D was deemed to be 0.04 utility points, as defined in the literature.17 Physical activity was evaluated with the International Physical Activity Questionnaire (IPAQ).18
Statistical analysis
Data were analysed in R 3.6.3 (R Foundation for Statistical Computing). Based on pilot data for an initial sample of 30 patients attending the RACPC, the expected mean absolute cardiovascular risk was 13% (standard deviation [SD], 4%). To detect a difference in absolute cardiovascular risk between control and intervention groups of 2 percentage points — a systematic review19 found an absolute difference of between 0.2 and 2.0 percentage points, and our methodology more closely reflected strategies that achieved greater risk reductions — we calculated that a sample size of 126 participants (63 per group) was required (α = 0.05; β = 0.2). As this was the first study of its kind in a chest pain clinic, however, it was difficult to confidently predict the effect size; further, we expected that about 15% of participants would be excluded by cardiac diagnoses at baseline assessment, and that 10% would be lost to follow‐up. We therefore aimed to recruit at least 200 participants.
Data are summarised as means with SDs or medians with interquartile ranges (IQRs). Data for categorical variables are summarised as numbers and proportions. Inter‐group differences in continuous baseline variables were assessed in two‐sample t tests or Wilcoxon rank‐sum tests; intra‐group differences between baseline and follow‐up were assessed in paired t tests, and inter‐group differences in outcomes in baseline‐adjusted analysis of covariance for continuous variables. The normality of distribution of model residuals was assessed in quantile‐quantile plots. Smoking cessation at follow‐up was assessed as relative risk, and the inter‐group difference evaluated by log binomial regression, adjusted for baseline smoking status. P < 0.05 was deemed statistically significant (P < 0.006 for secondary endpoints after Bonferroni adjustment), and 95% confidence intervals (CIs) are presented for model results.
Ethics approval
The study was approved by the University of Tasmania Human Research Ethics Committee (reference, H0014029). All participants provided written informed consent.
Results
During 1 July 2014 – 31 December 2017, 2120 patients were reviewed in the Royal Hobart Hospital RACPC; of 318 appropriate for inclusion, 220 patients (69%) consented to participate in our study. During clinical assessment and investigation, 28 patients (13%) were found to have cardiac causes for their chest pain and were excluded from the study. Three patients who did not attend follow‐up evaluation of risk factors were also excluded; the study sample therefore included 189 patients (Box 1). Participants were followed for a mean of 37.4 months (SD, 12.3 months). The baseline socio‐demographic and clinical characteristics of the two groups were similar (Box 2). Mean baseline absolute cardiovascular risk was lower for the 98 patients who declined participation than for the 220 who consented (11.7%; SD, 4.0% v 13.0%; SD, 4.2%). Risk scores for the 189 included patients (13.0%; SD, 4.3%) and the 28 excluded by a cardiac diagnosis at baseline assessment (12.9%; SD, 3.2%) were similar.
Change in absolute cardiovascular risk
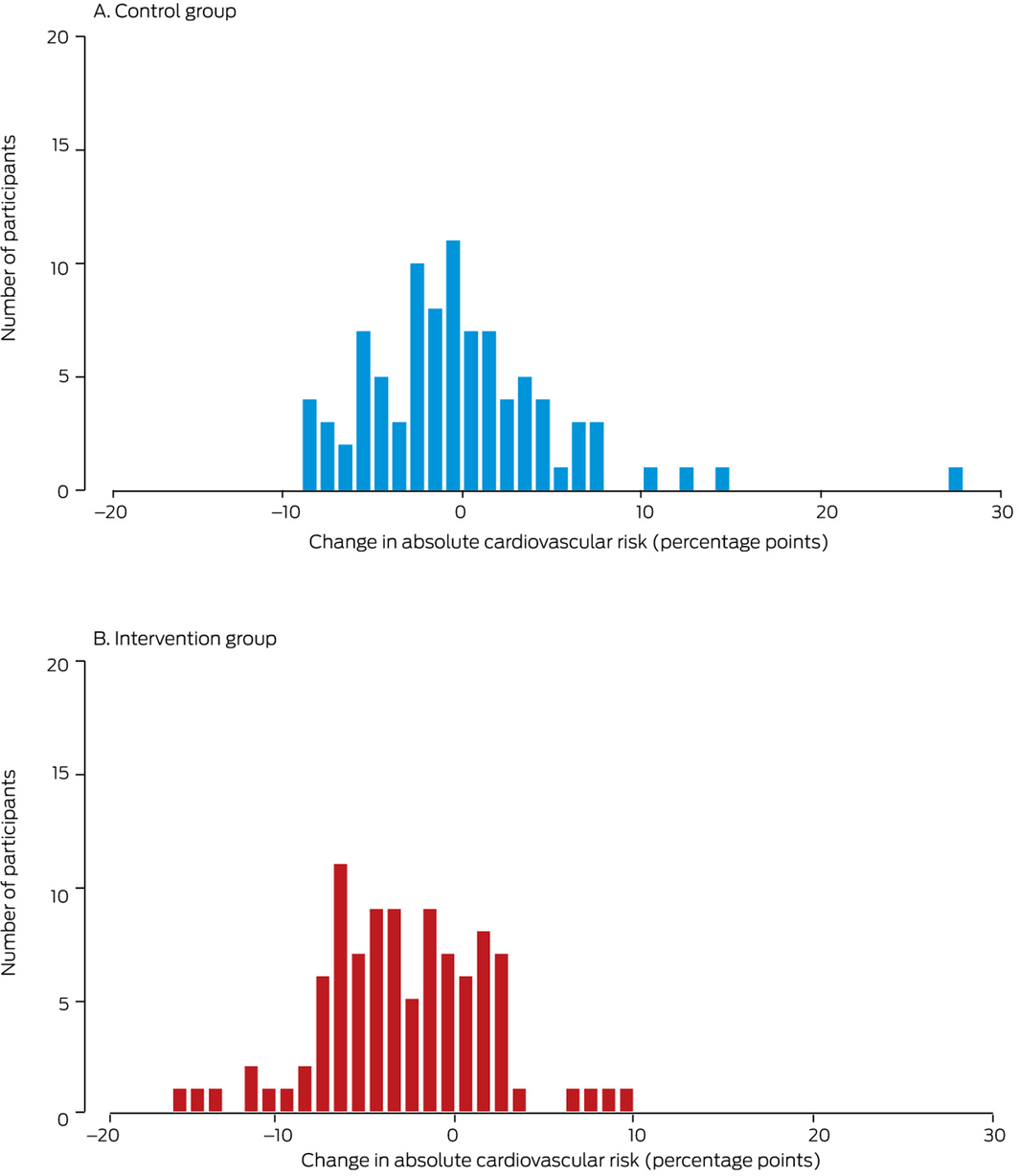
The mean change in risk was +0.4 percentage points (95% CI, –0.8 to 1.5 percentage points) for the control group and –2.4 percentage points (95% CI, –1.5 to –3.4 percentage points) for the intervention group (Box 3); the between‐group difference in change was 2.7 percentage points (95% CI, 1.3–4.1 percentage points).
The absolute cardiovascular risk value at follow‐up for one patient in the control group was identified as a significant outlier. Their estimated minimal absolute cardiovascular risk at baseline was 14% (total cholesterol, 3.6 mmol/L; HDL cholesterol, 0.8 mmol/L; systolic blood pressure, 147 mmHg), and they were taking a statin and three anti‐hypertensive agents. The patient subsequently disengaged from medical treatment and ceased all medications. At 21‐month follow up, his estimated absolute cardiovascular risk was 42% (total cholesterol, 7.2 mmol/L; HDL cholesterol, 0.7 mmol/L; systolic blood pressure, 205 mmHg). In a sensitivity analysis excluding this patient, the between‐group difference in primary endpoint was similar to that in the main analysis (intervention v control, –2.4 percentage points; 95% CI, –1.1 to –3.6 percentage points).
Changes in modifiable risk factors
Statistically significant improvements in smoking status, total cholesterol, LDL cholesterol, and systolic blood pressure were measured in both groups (Box 4). The changes in the control and intervention groups did not differ statistically significantly (Box 5).
Clinical events
Three patients (one in the control group, two in the intervention group) had myocardial infarctions by the time of follow‐up; there were no strokes or cardiac deaths. The three patients who withdrew from the study experienced no major adverse cardiovascular events.
Guideline‐based therapies
The increase in use of guideline‐based therapies was similar in the two groups. The use of lipid‐lowering therapy increased by 6 percentage points in the control group and by 5 percentage points in the intervention group. The use of anti‐hypertensive therapy increased by 4 percentage points in the control group and by 7 percentage points in the intervention group (Box 6).
Quality of life and physical activity
The SF‐36 questionnaire was completed by and SF‐6D health state utility values generated for 181 patients at baseline (96%; 93 intervention, 88 control patients) and for 125 patients at follow‐up (61%; 63 intervention, 62 control patients). The median change in health state utilities (improvement in quality of life) was clinically meaningful for both groups, and the between‐group difference favoured the intervention group (intervention, 0.16 utility points; control, 0.10 utility points; difference, 0.06 utility points).
The IPAQ was completed by 181 patients at baseline (96%; 93 intervention, 88 control), and 105 patients at follow‐up (56%; 56 intervention, 49 control). Self‐reported physical activity increased to a similar extent in the control (increase, 751 metabolic equivalents of task [MET] min/week; SD, 3222 MET min/week) and intervention groups (increase, 706 MET min/week; SD, 4563 MET min/week).
Discussion
Our principal finding was that an absolute cardiovascular risk‐based discussion with patients attending a chest pain clinic and implementation of an individualised risk factor management strategy significantly improved 5‐year cardiovascular risk scores over a period of at least 12 months.
Both patients and doctors estimate cardiovascular risk imprecisely,13 particularly patients with high risk.15 Optimistic bias may be a significant barrier to successful strategies for averting cardiovascular events. Improving patients’ appreciation of cardiovascular risk may facilitate adoption of preventive actions and activities.13
We sought to determine the effect of discussing absolute cardiovascular risk scores and developing corresponding strategies with patients attending our chest pain clinic. Our study was not powered to detect the influence of the intervention on individual modifiable risk factors, but the significant reduction in overall risk scores was accompanied by changes in several risk factors. The effect size was similar to that reported for other interventions designed to improve patients’ understanding of cardiovascular risk, but only in people who were offered education and counselling as well as notification of their risk scores.19
Previous studies had found that prescribing of guideline‐based therapies increased when absolute risk scores were documented during consultations.20 We found that the proportions of patients taking lipid‐lowering or anti‐hypertensive medications increased between baseline and follow‐up, but the magnitude of the changes was similar for the intervention and control groups. This is possibly because we excluded patients from groups with clinically determined high cardiovascular risk, as absolute risk calculations, and therefore NVDPA‐recommended pathways, are not appropriate for these patients. The mean 5‐year risk scores for included patients at baseline corresponded to intermediate cardiovascular risk, for which a lifestyle modification strategy is initially appropriate, with pharmacotherapy reserved for patients who do not achieve clinical risk factor targets.9 The greater reduction in mean risk score for the intervention group, without significantly larger changes in pharmacotherapy than the control group, suggests that intervention patients were more likely to adopt recommended lifestyle changes. The significantly greater improvement in quality of life further suggests positive lifestyle changes.
The reduction in mean risk score for the intervention group was associated with improvements in modifiable cardiovascular event risk factors. The magnitude of risk reduction would be clinically important if applied to a larger group of adults with moderate to high cardiovascular risk.19 Improvements in cardiovascular risk factors have been associated with subsequently reduced prevalence of cardiovascular disease and more favourable measures of cardiovascular structure and function.21 It should be noted, however, that a clear association between positive changes in risk score and subsequent adverse clinical events has not been established.22,23
The number of adverse clinical events in our study was low, reflecting the duration of follow‐up and a study group that excluded people with active cardiovascular disease.
Limitations
This study was conducted in a chest pain clinic; the outcomes may not be applicable outside this setting. A substantial number of patients declined participation, and we may have selected participants more receptive to risk factor modification. Intervention group patients were provided with some cardiovascular risk counselling by a registered nurse with cardiac rehabilitation experience, and the benefit of the intervention may be reduced if provided by physicians alone; however, in many settings it may be possible to provide combined nurse and physician counselling. We endeavoured to reduce observation bias by including a randomly allocated control group. The study was necessarily open label, but investigator bias was reduced by blinded endpoint assessment.
Conclusion
An absolute cardiovascular risk‐guided, individualised, pro‐active risk factor management strategy applied opportunistically in a chest pain clinic significantly improved 5‐year absolute cardiovascular risk scores. Our study provides further evidence that informing patients of their risk scores, and educating them about preventive measures, can significantly improve their cardiovascular risk profiles.
Box 2 – Baseline demographic and clinical characteristics of the study participants
|
|
Control group |
Intervention group |
|||||||||||||
|
|
|||||||||||||||
|
Number of participants |
91 |
98 |
|||||||||||||
|
Follow‐up (months), mean (SD) |
37.8 (13.0) |
37.2 (11.8) |
|||||||||||||
|
Age (years), mean (SD) |
59.0 (8.1) |
59.5 (7.9) |
|||||||||||||
|
Sex (men) |
71 (78%) |
66 (67%) |
|||||||||||||
|
Treated hypertension |
34 (37%) |
47 (48%) |
|||||||||||||
|
Diabetes mellitus |
12 (13%) |
17 (17%) |
|||||||||||||
|
Current smoking |
48 (53%) |
49 (50%) |
|||||||||||||
|
Treated dyslipidaemia |
21 (23%) |
35 (36%) |
|||||||||||||
|
Family history |
25 (28%) |
27 (28%) |
|||||||||||||
|
|
|||||||||||||||
|
SD = standard deviation. |
|||||||||||||||
Box 3 – Changes in individual absolute cardiovascular risk scores between baseline and follow‐up assessments, by study arm
Box 4 – Change in absolute cardiovascular risk and modifiable risk factors between baseline assessment and follow‐up (minimum, 12 months), by treatment group
|
|
Control |
Intervention |
|||||||||||||
|
|
Baseline |
Follow‐up |
P |
Baseline |
Follow‐up |
P |
|||||||||
|
|
|||||||||||||||
|
Primary endpoint |
|
|
|
|
|
|
|||||||||
|
5‐year absolute risk (%), mean (SD) |
12.8 (4.3) |
13.1 (6.3) |
0.52 |
13.1 (4.4) |
10.7 (5.0) |
< 0.001 |
|||||||||
|
Secondary endpoints |
|
|
|
|
|
|
|||||||||
|
Total cholesterol (mmol/L), mean (SD) |
5.5 (0.9) |
5.2 (1.1) |
0.008 |
5.5 (1.1) |
5.0 (1.2) |
< 0.001 |
|||||||||
|
LDL‐C (mmol/L), mean (SD) |
3.4 (1.0) |
3.1 (1.0) |
0.001 |
3.4 (1.0) |
2.8 (1.1) |
< 0.001 |
|||||||||
|
HDL‐C (mmol/L), mean (SD) |
1.2 (0.3) |
1.2 (0.3) |
0.61 |
1.2 (0.3) |
1.2 (0.4) |
0.10 |
|||||||||
|
Systolic blood pressure (mmHg), mean (SD) |
140.1 (13.9) |
136.0 (15.9) |
0.04 |
142.8 (14.5) |
134.6 (14.7) |
< 0.001 |
|||||||||
|
Body mass index (kg/m2), mean (SD) |
29.6 (5.6) |
29.6 (5.8) |
0.64 |
31.7 (5.6) |
31.1 (5.7) |
0.34 |
|||||||||
|
Current smoker |
48 (53%) |
41 (45%) |
< 0.001 |
49 (50%) |
33 (34%) |
< 0.001 |
|||||||||
|
|
|||||||||||||||
|
HDL‐C = high‐density lipoprotein cholesterol; LDL‐C = low‐density lipoprotein cholesterol; SD = standard deviation. |
|||||||||||||||
Box 5 – Adjusted between‐group difference in changes in absolute cardiovascular risk and modifiable risk factors between baseline assessment and follow‐up (minimum, 12 months), intervention v control
|
|
Between‐group difference (percentage points) (95% CI) * |
||||||||||||||
|
|
|||||||||||||||
|
Primary endpoint |
|
||||||||||||||
|
5‐year absolute risk |
2.70 (1.32 to 4.09) |
||||||||||||||
|
Secondary endpoints |
|
||||||||||||||
|
Total cholesterol (mmol/L) |
0.27 (–0.02 to 0.56) |
||||||||||||||
|
LDL‐C (mmol/L) |
0.28 (0.02 to 0.54) |
||||||||||||||
|
HDL‐C (mmol/L) |
–0.03 (–0.09 to 0.02) |
||||||||||||||
|
Systolic blood pressure (mmHg) |
2.28 (–1.92 to 6.49) |
||||||||||||||
|
Body mass index (kg/m2) |
0.39 (–0.65 to 1.43) |
||||||||||||||
|
|
Rate ratio (95% CI) † |
||||||||||||||
|
Smoker status |
1.96 (0.93–4.15) |
||||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; HDL‐C = high‐density lipoprotein cholesterol; LDL‐C = low‐density lipoprotein cholesterol. * Baseline‐adjusted analysis of variance. † Log binomial regression, adjusted for baseline smoking. |
|||||||||||||||
Received 21 June 2020, accepted 22 September 2020
- J Andrew Black1,2
- Julie A Campbell1
- Serena Parker2
- James E Sharman1
- Mark R Nelson3
- Petr Otahal1
- Garry Hamilton4
- Thomas H Marwick5
- 1 Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS
- 2 Royal Hobart Hospital, Hobart, TAS
- 3 University of Tasmania, Hobart, TAS
- 4 Austin Health, Melbourne, VIC
- 5 Baker IDI Heart and Diabetes Institute, Melbourne, VIC
We gratefully acknowledge grants from the Tasmanian Community Fund, the virtual Tasmanian Academic Health Sciences Precinct, and the Royal Hobart Hospital Research Foundation. We thank the staff and patients of the Royal Hobart Hospital who used or worked in the Rapid Access Chest Pain Clinic, as well as the National Heart Foundation for their support. We particularly appreciate the assistance of Bec Lane, Liam Kelleher, Roslyn Giles, Cathy MacIntosh, Vicki O’May, Teresa Grabek, Therese Hudson and Amanda Conroy (cardiology department, Royal Hobart Hospital) and Stephanie Pitney (smoking cessation clinic, Royal Hobart Hospital) with patient follow‐up.
No relevant disclosures.
- 1. Cullen L, Greenslade J, Merollini K, et al. Cost and outcomes of assessing patients with chest pain in an Australian emergency department. Med J Aust 2015; 202: 427–432. https://www.mja.com.au/journal/2015/202/8/cost-and-outcomes-assessing-patients-chest-pain-australian-emergency-department
- 2. Black JA, Cheng K, Flood JA, et al. Evaluating the benefits of a rapid access chest pain clinic in Australia. Med J Aust 2019; 210: 321–325. https://www.mja.com.au/journal/2019/210/7/evaluating-benefits-rapid-access-chest-pain-clinic-australia
- 3. Tonkin A, Barter P, Best J, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement on lipid management 2005. Heart Lung Circ 2005; 14: 275–291.
- 4. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2889–2934.
- 5. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357.
- 6. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–188.
- 7. Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991; 121: 293–298.
- 8. Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation 1991; 83: 356–362.
- 9. National Vascular Disease Prevention Alliance. Guidelines for the management of absolute cardiovascular disease risk. 2012. http://www.cvdcheck.org.au/links-to-resources/36-for-health-professionals/31-footnotes (viewed June 2020).
- 10. Jackson R, Lawes CM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365: 434–441.
- 11. Kannel WB. Some lessons in cardiovascular epidemiology from Framingham. Am J Cardiol 1976; 37: 269–282.
- 12. Manuel DG, Lim J, Tanuseputro P, et al. Revisiting Rose: strategies for reducing coronary heart disease. BMJ 2006; 332: 659–662.
- 13. Webster R, Heeley E. Perceptions of risk: understanding cardiovascular disease. Risk Manag Healthc Policy 2010; 3: 49–60.
- 14. Ahmad T, Mora S. Providing patients with global cardiovascular risk information: is knowledge power? Arch Intern Med 2010; 170: 227–228.
- 15. Liew SM, Lee WK, Khoo EM, et al. Can doctors and patients correctly estimate cardiovascular risk? A cross-sectional study in primary care. BMJ Open 2018; 8: e017711.
- 16. Norman R, Viney R, Brazier J, et al. Valuing SF-6D health states using a discrete choice experiment. Med Decis Making 2014; 34: 773–786.
- 17. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005; 14: 1523–1532.
- 18. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395.
- 19. Sheridan SL, Viera AJ, Krantz MJ, et al. The effect of giving global coronary risk information to adults: a systematic review. Arch Intern Med 2010; 170: 230–239.
- 20. Hall LM, Jung RT, Leese GP. Controlled trial of effect of documented cardiovascular risk scores on prescribing. BMJ 2003; 326: 251–252.
- 21. Shah AM, Claggett B, Folsom AR, et al. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation 2015; 132: 1979–1789.
- 22. van Sloten TT, Tafflet M, Perier MC, et al. Association of change in cardiovascular risk factors with incident cardiovascular events. JAMA 2018; 320: 1793–1804.
- 23. Usher-Smith JA, Silarova B, Schuit E, et al. Impact of provision of cardiovascular disease risk estimates to healthcare professionals and patients: a systematic review. BMJ Open 2015; 5: e008717.





Abstract
Objectives: To assess the efficacy of a pro‐active, absolute cardiovascular risk‐guided approach to opportunistically modifying cardiovascular risk factors in patients without coronary ischaemia attending a chest pain clinic.
Design: Prospective, randomised, open label, blinded endpoint study.
Setting: The rapid access chest pain clinic of Royal Hobart Hospital, a tertiary hospital.
Participants: Patients who presented to the chest pain clinic between 1 July 2014 and 31 December 2017 who had intermediate to high absolute cardiovascular risk scores (5‐year risk ≥ 8%). Patients with known cardiac disease or from groups with clinically determined high risk of cardiovascular disease were excluded.
Main outcome measures: The primary endpoint was change in 5‐year absolute risk score (Australian absolute risk calculator) at follow‐up (at least 12 months after baseline assessment). Secondary endpoints were changes in lipid profile, blood pressure, smoking status, and body mass index, and major adverse cardiovascular events.
Results: The mean change in risk at follow‐up was +0.4 percentage points (95% CI, –0.8 to 1.5 percentage points) for the 98 control group patients and –2.4 percentage points (95% CI, –1.5 to –3.4 percentage points) for the 91 intervention group patients; the between‐group difference in change was 2.7 percentage points (95% CI, 1.2–4.1 percentage points). Mean changes in lipid profile, systolic blood pressure, and smoking status were larger for the intervention group, but not statistically different from those for the control group.
Conclusions: An absolute cardiovascular risk‐guided, pro‐active risk factor management strategy employed opportunistically in a chest pain clinic significantly improved 5‐year absolute cardiovascular risk scores.
Trial registration: Australia New Zealand Clinical Trial Registry, ACTRN12617000615381 (retrospective).