Acute rheumatic fever (ARF) is an autoimmune disease triggered in some children and young adults by infection with group A streptococci.1 Repeated or severe ARF leads to rheumatic heart disease (RHD), with high morbidity and mortality. Group A streptococcal infection risk is associated with socio‐economic factors such as household crowding.2 High rates occur in Australian Aboriginal and Torres Strait Islander populations, especially in rural or remote settings. Prevalence estimates for definite RHD in Australian children range from < 1 per 1000 population in low risk children, to 333 to 504 per 1000 people in high risk populations. High rates of disease also occur among Māori and Pacific Islander populations.5
Given this high burden of disease in Australian subpopulations, yet rarity in the broader population, clinical practice guidelines are essential. Australian ARF and RHD guidelines were first produced in 20076 and revised in 2012.7 The 2020 guideline, developed in accordance with the National Health and Medical Research Council standards for guidelines8 by RHDAustralia (the national support unit for ARF and RHD), builds on these and incorporates new evidence from trials and other research, new medication options — such as expanded roles for corticosteroids, and use of non‐vitamin K antagonist oral anticoagulants — and new expert consensus opinion including revised parameters for ARF and RHD diagnosis. The guideline9 is freely available online, accompanied by a video summary of changes, key information, useful tables and figures, an app for smart devices containing a condensed version, and an interactive ARF diagnosis calculator.10 All electronic resources align with the 2020 edition and the 2015 American Heart Association (AHA) revised Jones criteria for diagnosis of ARF11 to ensure consistency and best practice.
Methods
A guideline steering committee was formed comprising RHDAustralia members and partners, ARF and RHD experts, and Aboriginal and Torres Strait Islander advisors. The steering committee provided high level strategic direction and advice, content support and endorsement of the final version.
RHDAustralia's Senior Aboriginal Cultural Advisor led a review of all content. A sociocultural framework highlighted social and emotional wellbeing, and ensured that recommendations adhered to culturally safe practice. An Aboriginal and Torres Strait Islander advisory group provided expert cultural advice, with consumer input from community members (Champions4Change12). Insights from the Champions introduce each chapter, such as: “You need to understand the community and the problems that they are facing and then, and only then, you can help them to get rid of RHD”.9
A targeted health workforce survey was conducted to inform the format and scope of the new edition. The 196 respondents (53% urban, 18% rural, 29% remote) indicated that a freely available digital version as well as print copies was desired, with a quick guide format as additional detail. Each chapter structure therefore comprises a key information section followed by an evidence‐based discussion and, where relevant, case studies.
The steering committee developed chapter headings and invited multidisciplinary experts (Indigenous and non‐Indigenous medical, nursing, research and allied health specialties) from among Australian and New Zealand topic authorities (Supporting Information, Table 1). Authors reviewed relevant chapters from the 2012 edition (unless developing a new chapter), conducted literature reviews using MEDLINE and PubMed Central, and considered in‐process citations, research underway and grey literature. The lived experience of ARF and RHD was represented through patient stories and case studies.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system13 was applied by writing groups where appropriate. Quality of evidence was classified from A (high) to D (very low) and the strength of the recommendations graded as 1 (strong) or 2 (weak). For example, GRADE 1A indicates that the recommendation should be applied to most patients without reservation, while GRADE 2D indicates that evidence is lacking but expert consensus weakly supports the recommendation. New recommendations not firmly supported by evidence or where evidence was contentious were discussed until consensus was reached, or until an acceptable majority position was obtained considering the available evidence. The aim was to present feasible rather than highly aspirational guidance for ARF and RHD control in such cases; examples include definition of ARF risk groups, duration of secondary prophylaxis, and recommendations for community echocardiogram screening for active case finding in high risk communities. Changes from the previous edition are summarised in Box 1.
Feedback was invited from multidisciplinary content experts in Australia and New Zealand, and recommendations were incorporated where appropriate. Endorsement was sought from peak health policy, advocacy and training organisations (Supporting Information, Table 2). The editors reviewed the semi‐final draft to ensure inclusion of any recently published or in‐press literature, consistency across chapters, clarity for practitioners, and alignment with other Australian and international guidelines. Where recommendations departed from other local guidelines (eg, Australian Therapeutic Guidelines: Antibiotic
[ https://www.tg.org.au] or the Central Australian Rural Practitioners Association Standard Treatment Manual16), this was communicated to respective editors to encourage alignment in their next edition.
Culture and workforce
The guideline is underpinned by a strong focus on the provision of culturally safe care, in line with national recommendations.17 Cultural safety requires health care providers and institutions to recognise their own culture and ameliorate approaches which diminish, demean or disempower the cultural identities and wellbeing of patients.18 A socio‐ecological model was developed for the guideline (Box 2), describing influences shaping Aboriginal and Torres Strait Islander peoples’ health care interactions. The value of fostering a strong Aboriginal health workforce in delivering care is highlighted to support effective client engagement.19,20
Burden of disease
We developed a new chapter on burden of disease, using original data from the End RHD in Australia Study of Epidemiology (ERASE) Project,21 Australian Institute of Health and Welfare data22 and other sources. Since the early 1990s, ARF has occurred almost exclusively in young Aboriginal and Torres Strait Islander people (94% of cases; 33% affecting 5–14‐year‐olds). The recognised female predominance in rates of ARF (56%) and RHD (61%) is reiterated.22
Recommendations
Primordial prevention and social determinants of ARF
Primordial prevention reduces community‐based risk factors to prevent the occurrence of a disease. ARF is attributable to social determinants of health, including quality of housing, level of household occupancy, and access to health hardware including washing facilities.2,23 The Nine Healthy Living Practices24,25 are simple recommendations to reduce the risk of injury, communicable diseases and environmental diseases in household settings. They are used as the framework for this chapter. Each Practice was reviewed and the evidence graded regarding their likely association with reducing streptococcal infection at community level. The two practices for which the evidence is graded as strong are “washing people” and “reducing negative impacts of overcrowding”.
Primary prevention
People at high risk of ARF (Box 3) require empirical antibiotic treatment for sore throat, with penicillin the first line choice (GRADE 1B).26,27,28 Impetigo caused by group A streptococcus is very common among Aboriginal and Torres Strait Islander children living in remote areas, with almost one in two affected at any time.29 Identification, treatment and prevention of group A streptococcal skin infections may help reduce ARF burden (GRADE 1B).30,31,32,33,34 Group A streptococcal skin infections should be treated with cotrimoxazole orally or benzathine benzylpenicillin G intramuscularly (GRADE 1A).35 Dosing regimens are provided in the full guideline (Table 5.3).9
ARF diagnosis
There is now alignment between Australian diagnostic criteria for ARF and the AHA revised Jones criteria11 (Box 4), outlining differences for high and low risk populations (Box 3). The changes to diagnostic criteria in low risk groups include a higher temperature (≥ 38.5°C rather than ≥ 38°C), higher erythrocyte sedimentation rate (≥ 60 mm/h rather than ≥ 30 mm/h), and echocardiographic evidence of valvulitis with carditis. A combination of major and minor criteria is used to diagnose ARF; major criteria including arthritis, carditis, chorea and skin manifestations are strongly associated with ARF, while minor criteria such as fever and raised inflammatory markers support the diagnosis. For all populations, definite recurrent ARF now requires two major, or one major and two minor, or three minor criteria, rather than two major, or one major and one minor, or three minor criteria. Alignment with the AHA is important to promote a consistent approach to ARF diagnosis globally, and the changes also improve specificity of ARF diagnosis, especially in low risk populations where ARF is very uncommon.
All patients with suspected ARF should be hospitalised, investigated with electrocardiography and echocardiography, and have differential diagnoses excluded (GRADE 1B). Each episode should be categorised as initial or recurrent ARF, with certainty of diagnosis indicated as definite, probable or possible:
- definite ARF meets revised Jones criteria with alternative diagnoses excluded;
- probable ARF is an acute presentation not fulfilling criteria, missing one major or one minor criterion or lacking evidence of preceding streptococcal infection, but where ARF is still considered the most likely diagnosis; and
- possible ARF applies to the same presentation type as probable ARF, but where ARF is considered uncertain but cannot be ruled out.
ARF management
The pillars of ARF management are eradication of the inciting group A streptococcal infection using penicillin (or an alternative if allergic to penicillin) and management of symptoms with analgesic–antipyretic agents as needed (GRADE 1B). The guideline discusses the use of corticosteroids as a potential disease‐modifying agent in severe rheumatic carditis (GRADE 2B), and to reduce severity of Sydenham chorea (GRADE 2B).
For definite ARF, a priority grade based on the severity of any accompanying RHD should be assigned, using a revised priority classification (Supporting Information, Table 3). The time since ARF and the severity of RHD determine the duration of secondary prophylaxis (Box 5) and the priority grade determines frequency of reviews and echocardiograms. People diagnosed with ARF must be notified to the local public health unit in accordance with Australian state and territory legislation and be registered with the jurisdictional RHD control program (GRADE 1B).
Diagnosis of RHD
The guideline provides more detail on the use of echocardiogram in accordance with World Heart Federation recommendations on echocardiographic diagnosis of RHD,36 which provide criteria distinguishing pathological RHD from physiological changes (GRADE 1B). Exercise testing or stress echocardiography is recommended when severity of symptoms and echocardiographic findings are discordant (GRADE 1B). Transoesophageal echocardiography may help in planning surgical intervention (GRADE 1B).
RHD is also notifiable in Western Australia, South Australia, Northern Territory, Queensland, and New South Wales (RHD for people aged < 35 years).5
Screening for RHD
Population screening for RHD may provide more accurate estimates of disease burden and an opportunity to initiate management for people with previously unrecognised RHD. Population‐based screening using auscultation, inaccurate for detecting RHD, is not recommended (GRADE 1A). Screening using echocardiography can accurately detect previously undiagnosed RHD (GRADE 1A). Echocardiographic screening procedures have evolved using different technologies and operators with varying levels of expertise.4 Echocardiographic screening for RHD meets some but not all public health criteria for community screening.37 The disease does place a significant burden on at‐risk populations, there is a latent stage that can be identified, and there is treatment in the form of secondary prophylaxis and cardiological or surgical intervention. However, the impact of secondary prophylaxis on the trajectory of screen‐detected RHD is not yet defined, and feasible community screening tools have thus far demonstrated inadequate sensitivity and specificity.4 While there remains insufficient evidence to recommend routine population‐level echocardiographic screening for RHD in Australia as a method of disease detection and control (GRADE 2B), it is recognised that echocardiographic community screening is valuable under specific circumstances such as clusters of ARF or suspected extreme rates of RHD.4
Secondary prophylaxis ARF
Secondary prophylaxis comprises regular administration of antibiotics after diagnosis of ARF or RHD to prevent future group A streptococcal infections and ARF recurrence. Group A streptococcus does not develop resistance to penicillin, although one instance of acquisition of reduced ampicillin susceptibility has been reported.38 Long acting benzathine benzylpenicillin G delivered every 28 days is the first line recommendation for ARF prophylaxis (GRADE 1B). Previously, secondary prophylaxis was recommended in Australia for at least 10 years after the most recent episode of ARF or until 21 years of age, whichever comes later. The 2020 guideline recommends secondary prophylaxis for 5 years after the most recent episode of ARF or until 21 years of age if there has been no acute cardiac involvement evident on electrocardiograph or echocardiogram during ARF, and follow‐up and end‐of‐treatment echocardiograms confirm ongoing absence of valvular involvement (Box 5). This is more aligned with international guidelines39,40 and is supported by Australian register data.
Management of RHD
Every patient with RHD should have access to specialist paediatric or adult cardiology services, and coordinated transition from paediatric to adult care (GRADE 2A). Non‐vitamin K antagonist oral anticoagulants can be used in patients with RHD‐related atrial fibrillation or elevated CHA2DS2‐VA (congestive heart disease, hypertension, age, diabetes, stroke, vascular) score, even if valvular disease is present, provided there is no mitral stenosis of moderate or greater severity and no mechanical valve in situ (GRADE 2B). For patients with moderate or severe mitral stenosis and atrial fibrillation, vitamin K antagonists (eg, warfarin) currently remain the only indicated oral anticoagulant (GRADE 1B).41
Surgical decision making must take into consideration a patient's personal, social and cultural situation. Early engagement of a multidisciplinary team is essential to determine the appropriate choice and timing of intervention. Surgical options include repair, bioprosthetic or mechanical valve replacement, and transcatheter valve replacement. Key considerations are the patient's age, risks of anticoagulation, anticipated adherence, plans for future pregnancy, and durability of valve repair and prosthesis.
Antibiotic prophylaxis for endocarditis prevention with amoxycillin (first line) is recommended in all people with RHD undergoing invasive procedures as defined in Table 11.5 of the guideline9 (GRADE 1C).
Females with RHD
About 61% of RHD cases in Australia occur in females.5 Women with moderate or greater mitral stenosis, severe mitral or aortic regurgitation, severe aortic stenosis, pulmonary hypertension or heart failure are at high risk of cardiac events during pregnancy and have an elevated chance of adverse fetal outcomes. A left ventricular ejection fraction < 30% or reduced systolic function with New York Heart Association class III–IV symptoms is associated with high maternal morbidity or mortality, and pregnancy is strongly discouraged.42 Conversely, selected women with mild RHD can safely conceive and have children. In 2–3% of annually recorded pregnancies among Aboriginal women in the Northern Territory, the women have RHD. Women with mild RHD may be able to give birth on Country, an important cultural practice for many Aboriginal and Torres Strait Islander people.43
Pre‐conception diagnosis of RHD is critical to optimise management including potential surgery. Long acting, reversible contraceptives (eg, intra‐uterine contraceptive devices, etonogestrel implants) are recommended for women who agree to avoid pregnancy after advice. Oestrogen‐containing contraceptives are associated with elevated risk of thrombosis (GRADE 1A) and should be avoided if additional thrombosis risks are present.
A pregnant woman in a high risk group for ARF and RHD who presents with breathlessness, orthopnoea, wheeze or worsening fatigue should be investigated with an echocardiogram (GRADE 1A). Normal vaginal delivery is generally preferred for women with RHD. Epidural anaesthesia (after appropriately timed, short term cessation of any anticoagulation) may be indicated to reduce tachycardia and hypertension that can precipitate acute heart failure during delivery.
RHD control programs
Comprehensive RHD control programs can provide effective approaches to reducing the burden of RHD (GRADE 1B). RHD control programs in Australia maintain register and recall systems for secondary prophylaxis and optimum clinical management; support patient care and education about ARF and RHD through workforce education and training; promote primary prevention aimed at preventing initial episodes of ARF; and provide jurisdiction‐wide data for epidemiological reporting ( https://www.rhdaustralia.org.au/control-programs).
New technologies
Research underway in Australasia aims to discover alternatives to or improvement in delivery of benzathine benzylpenicillin G, develop a group A streptococcus vaccine, and develop a diagnostic test for ARF.
Conclusion
The 2020 ARF and RHD guideline places person and culture at the centre of care and synthesises the current evidence to provide expert clinical guidance from prevention through to tertiary care.
Box 1 – Summary of changes from the 2012 edition
|
Chapter |
Changes |
||||||||||||||
|
|
|||||||||||||||
|
Primary prevention |
|
||||||||||||||
|
Diagnosis of ARF |
|
||||||||||||||
|
Management of ARF |
|
||||||||||||||
|
Diagnosis of RHD |
|
||||||||||||||
|
Secondary prophylaxis |
|
||||||||||||||
|
Management of RHD |
|
||||||||||||||
|
Women and girls with RHD |
|
||||||||||||||
|
New chapters |
|
||||||||||||||
|
|
|||||||||||||||
|
ARF = acute rheumatic fever; BPG = benzathine benzylpenicillin G; CHA2DS2‐VA = congestive heart failure, hypertension, age, diabetes, stroke, vascular, age; ESR = erythrocyte sedimentation rate; RHD = rheumatic heart disease. |
|||||||||||||||
Box 2 – Socio‐ecological model underpinning the guidelines*
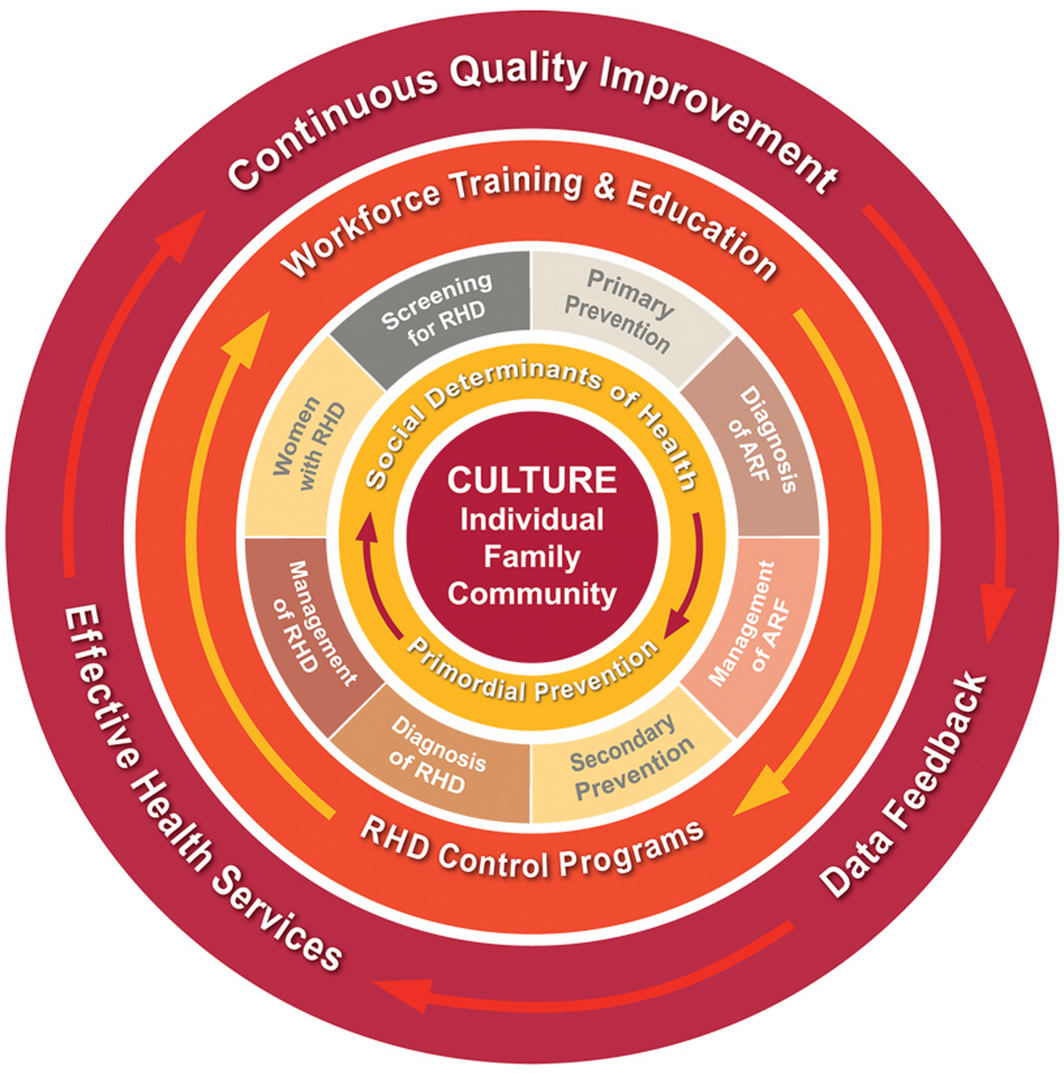
* Reproduced with permission from Menzies School of Health Research, Charles Darwin University, Darwin, Australia, which holds copyright: https://www.rhdaustralia.org.au/resources/2020-guidelineprevention-diagnosis-and-management-acute-rheumatic-fever-and-rheumatic.9
Box 3 – Risk groups for acute rheumatic fever (ARF) and rheumatic heart disease (RHD)*
|
Risk |
Setting |
||||||||||||||
|
|
|||||||||||||||
|
High risk |
|
||||||||||||||
|
May be high risk |
|
||||||||||||||
|
Additional considerations which increase risk |
|
||||||||||||||
|
|
|||||||||||||||
|
* Reproduced with permission from Menzies School of Health Research, Charles Darwin University, Darwin, Australia, which holds copyright: https://www.rhdaustralia.org.au/resources/2020-guideline-prevention-diagnosis-and-management-acute-rheumatic-fever-and-rheumatic.9 † Populations where community ARF/RHD rates are known to be high; eg, ARF incidence > 30/100 000 per year in 5–14‐year‐olds or RHD all‐age prevalence > 2/1000. |
|||||||||||||||
Box 4 – 2020 Australian criteria for acute rheumatic fever (ARF) diagnosis*
|
|
High risk groups† |
Low risk groups |
|||||||||||||
|
|
|||||||||||||||
|
Definite initial episode of ARF |
|
||||||||||||||
|
Definite recurrent‡ episode of ARF in patient with documented history of ARF or RHD |
|
||||||||||||||
|
Probable or possible ARF (first episode or recurrence§) |
A clinical presentation in which ARF is considered a likely diagnosis but falls short in meeting the criteria by either:
|
||||||||||||||
|
Major manifestations |
|
|
|||||||||||||
|
Minor manifestations |
|
|
|||||||||||||
|
|
|||||||||||||||
|
CRP = C‐reactive protein; ECG = electrocardiogram; ESR = erythrocyte sedimentation rate; RHD = rheumatic heart disease. * Reproduced with permission from Menzies School of Health Research, Charles Darwin University, Darwin, Australia, which holds copyright: https://www.rhdaustralia.org.au/resources/2020-guideline-prevention-diagnosis-and-management-acute-rheumatic-fever-and-rheumatic.9 † High risk groups are those living in communities with high rates of ARF (incidence > 30/100 000 per year in 5–14‐year‐olds) or RHD (all‐age prevalence > 2/1000). Aboriginal and Torres Strait Islander peoples living in rural or remote settings are known to be at high risk. Data are not available for other populations but Aboriginal and Torres Strait Islander peoples living in urban settings, Māori and Pacific Islanders, and potentially immigrants from developing countries, may also be at high risk. ‡ Elevated or rising antistreptolysin O or other streptococcal antibody, or a positive throat culture or rapid antigen or nucleic acid test for group A streptococcal infection. § Recurrent definite, probable or possible ARF requires a time period of more than 90 days after the onset of symptoms from the previous episode of definite, probable or possible ARF. ¶ A definite history of arthritis is sufficient to satisfy this manifestation. Note that if polyarthritis is present as a major manifestation, polyarthralgia or aseptic monoarthritis cannot be considered an additional minor manifestation in the same person. ** Chorea does not require other manifestations or evidence of preceding group A streptococcal infection, provided other causes of chorea are excluded. †† Care should be taken not to label other rashes, particularly non‐specific viral exanthems, as erythema marginatum. ‡‡ ‡‡In high risk groups, fever can be considered a minor manifestation based on a reliable history (in the absence of documented temperature) if anti‐inflammatory medication has already been administered. §§ If polyarthritis is present as a major criterion, monoarthritis or arthralgia cannot be considered an additional minor manifestation. ¶¶ If carditis is present as a major manifestation, a prolonged PR interval cannot be considered an additional minor manifestation. |
|||||||||||||||
Box 5 – Recommended duration of secondary prophylaxisfor acute rheumatic fever (ARF) and rheumatic heart disease (RHD)*
|
Diagnosis |
Definition |
Duration of prophylaxis |
Conditions for ceasing prophylaxis† |
Timing of echocardiography after cessation‡ |
|||||||||||
|
|
|||||||||||||||
|
Possible ARF (no cardiac involvement) |
Incomplete features of ARF with normal echocardiogram and normal ECG§ throughout ARF episode |
12 months (then reassess) |
|
At 1 year |
|||||||||||
|
Probable ARF |
Highly suspected ARF with normal echocardiogram |
Minimum of 5 years after most recent episode of probable ARF, or until age 21 years (whichever is longer) |
|
At 1, 3 and 5 years |
|||||||||||
|
Definite ARF (no cardiac involvement) |
ARF with normal echocardiogram and normal ECG§ throughout ARF episode |
Minimum of 5 years after most recent episode of ARF, or until age 21 years (whichever is longer) |
|
At 1, 3 and 5 years |
|||||||||||
|
Definite ARF(with cardiac involvement) |
ARF with carditis or RHD on echocardiogram, or with atrioventricular conduction abnormality on ECG§ during ARF episode |
According to relevant RHD severity |
|||||||||||||
|
Borderline RHD (≤ 20 years of age only) |
Borderline RHD on echocardiogram without a documented history of ARF |
Not usually recommended¶ |
|
Medical review and repeat echocardiogram at 1, 3 and 5 years after diagnosis |
|||||||||||
|
Mild RHD†† |
Echocardiogram showing:
|
|
|
At 1, 3 and 5 years |
|||||||||||
|
Moderate RHD††,§§ |
Echocardiogram showing:
|
|
|
Initially every 12 months |
|||||||||||
|
Severe RHD§§,¶¶ |
Echocardiogram showing:
|
|
|
Initially every 6 months |
|||||||||||
|
|
|||||||||||||||
|
AV = atrioventricular; ECG = electrocardiogram. * Reproduced with permission from Menzies School of Health Research, Charles Darwin University, Darwin, Australia, which holds copyright: https://www.rhdaustralia.org.au/resources/2020-guideline-prevention-diagnosis-and-management-acute-rheumatic-fever-and-rheumatic.9 † All people receiving secondary prophylaxis require a comprehensive clinical assessment and echocardiogram before cessation. Risk factors including future exposure to high streptococcal burden environments need to be considered. ‡ Echocardiography may be more frequent based on clinical status and specialist review. § Normal ECG means no AV conduction abnormality during the ARF episode — including first degree heart block, second degree heart block, third degree (complete) heart block and accelerated junctional rhythm. ¶ Secondary prophylaxis may be considered in some circumstances, including family preference, family history of rheumatic heart valve surgery, or suspected retrospective history of ARF. If prophylaxis is commenced, consider ceasing after 1–3 years if no history of ARF and if echocardiographic features have resolved or not progressed to definite RHD. †† Prophylaxis may be considered for longer in women considering pregnancy who live in high risk circumstances for ARF. ‡‡ If diagnosed with mild or moderate RHD aged ≥ 35 years (without ARF), secondary prophylaxis is not required. §§ Rarely, moderate or severe RHD may improve on echocardiogram without valve surgery. In these cases, the conditions for ceasing prophylaxis can change to follow the most relevant severity category. For instance, if moderate RHD improves to mild on echocardiogram, recommendations for mild RHD can then be instigated. ¶¶ Risk of ARF recurrence is low in people aged ≥ 40 years; however, lifelong secondary prophylaxis is usually recommended for patients who have had, or are likely to need, heart valve surgery. ††† If diagnosed with severe RHD aged ≥ 40 years (without ARF), specialist input is required to determine the need for secondary prophylaxis. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.





Abstract
Introduction: Acute rheumatic fever (ARF) and rheumatic heart disease (RHD) cause significant morbidity and premature mortality among Australian Aboriginal and Torres Strait Islander peoples. RHDAustralia has produced a fully updated clinical guideline in response to new knowledge gained since the 2012 edition. The guideline aligns with major international ARF and RHD practice guidelines from the American Heart Association and World Heart Federation to ensure best practice. The GRADE system was used to assess the quality and strength of evidence where appropriate.
Main recommendations: The 2020 Australian guideline details best practice care for people with or at risk of ARF and RHD. It provides up‐to‐date guidance on primordial, primary and secondary prevention, diagnosis and management, preconception and perinatal management of women with RHD, culturally safe practice, provision of a trained and supported Aboriginal and Torres Strait Islander workforce, disease burden, RHD screening, control programs and new technologies.
Changes in management as a result of the guideline: Key changes include updating of ARF and RHD diagnostic criteria; change in secondary prophylaxis duration; improved pain management for intramuscular injections; and changes to antibiotic regimens for primary prevention. Other changes include an emphasis on provision of culturally appropriate care; updated burden of disease data using linked register and hospitalisations data; primordial prevention strategies to reduce streptococcal infection addressing household overcrowding and personal hygiene; recommendations for population‐based echocardiographic screening for RHD in select populations; expanded management guidance for women with RHD or ARF to cover contraception, antenatal, delivery and postnatal care, and to stratify pregnancy risks according to RHD severity; and a priority classification system for presence and severity of RHD to align with appropriate timing of follow‐up.