The known: In Australia, early stage prostate cancer is usually diagnosed by urologists in the private or public health systems. The major treatment options are radical prostatectomy, radiation therapy alone or after surgery, and active surveillance.
The new: We found substantial differences between private and public health services in the treatment choices made for men with prostate cancer, even after adjusting for age, tumour grade and comorbidity.
The implications: Our findings have diverse implications for patients, clinicians, and the health system, all of which should be taken into account when considering further investigation of or response to the differences we have identified.
The incidence of prostate cancer has increased in Australia over the past 20 years and is now the most frequently diagnosed cancer.1 Case‐finding by prostate‐specific antigen (PSA) blood testing is increasingly common for men aged 45 years or more, and they may proceed to biopsy if their PSA level is elevated.2 If cancer is detected, the choice of subsequent care — active surveillance, watchful waiting, androgen deprivation therapy, radiation therapy, or radical prostatectomy — depends on the tumour risk group, the patient's age, his preferences and those of his doctor, comorbid conditions, and the long term prognosis.
Many men with localised prostate cancer undergo active treatment, although the only two prospective randomised trials during the era of widespread PSA testing found that prostate‐specific and overall survival outcomes were equivalent for surgery, radiation therapy, and watchful waiting (despite some documented limitations).3,4 As active therapy has significant side effects, including urinary incontinence and erectile dysfunction, overtreatment should be avoided.5 Widespread PSA testing of asymptomatic men has contributed to high rates of overdiagnosis and overtreatment of people with low risk prostate cancer in Australia,6 but the use of radical treatment in such cases may be starting to decline, both here and overseas.7,8
The treatment preferred by a patient depends on his understanding of the prognosis and the risks and benefits of the options. His choice may be influenced by how the information is presented, and not all patients receive information adequate for making a fully informed decision.9 This can lead to decision regret, increased treatment costs, and psychological problems.10,11,12 With our ageing population and the increasing costs of prostate cancer treatment, these factors also have health economic implications.1
We therefore assessed prostate cancer treatments provided to Victorian men within 12 months of diagnosis with localised prostate cancer, comparing treatments for men diagnosed in private and public health services. We analysed population‐based cancer registry data linked to population‐based administrative hospital and radiation therapy data.
Methods
We identified Victorian men newly diagnosed with prostate cancer between 1 January 2011 and 31 December 2017, as recorded in the Victorian Cancer Registry (VCR). The Centre for Victorian Data Linkage linked the VCR dataset to the Victorian Admitted Episodes Dataset (VAED; 1 January 2010 – 31 December 2018) and the Victorian Radiotherapy Minimum Dataset (VRMDS; 1 January 2011 – 31 December 2018), applying iterative deterministic linkage with fuzzy matching on selected fields. The VAED contains demographic, administrative and clinical data for patients admitted to public or private health services in Victoria. Each admission episode includes up to 40 diagnostic and procedural codes (International Statistical Classification of Diseases and Related Health Problems, tenth revision, Australian modification [ICD‐10‐AM]; online Supporting Information, table 1) to describe the reasons for admission and the procedures performed. The VRMDS contains demographic, administrative and clinical data for admitted and non‐admitted patients treated in public and private Victorian radiation therapy facilities, including the primary diagnosis, the palliative or curative nature of the radiation course, and its anatomic target.
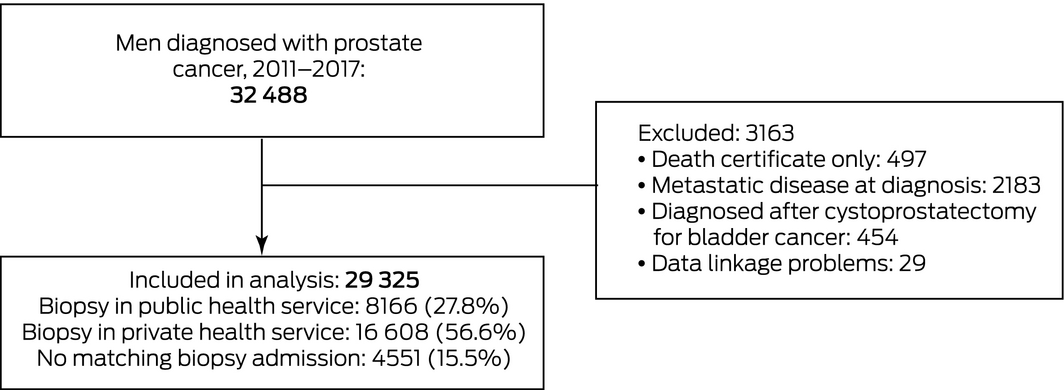
Men with prostate cancer diagnoses known only from death certificates were excluded, as were those with metastatic disease at diagnosis (health service admissions within four months of diagnosis with ICD‐10‐AM nodal [C77x] or metastatic disease codes [C78x, C79x]), and diagnoses following cystoprostatectomy for bladder cancer.
Men with localised prostate cancer were categorised as private or public patients according to the health service at which the prostate biopsy closest to the VCR‐defined diagnosis date was performed. Only biopsy‐related admissions within three months of the VCR‐defined diagnosis date were included; 97% of included biopsies were performed within three days of diagnosis.
Two treatment types within 12 months of biopsy or VCR diagnosis date were assessed:
- radical prostatectomy, with or without radiation therapy with curative intent (Supporting Information, table 1); and
- curative external beam radiation therapy alone: radiation therapy with curative intent. The primary target was either the prostate or pelvis, and a primary diagnosis of prostate cancer was coded in the VRMDS. Brachytherapy alone was excluded, as data from private centres is incomplete.
Data for androgen deprivation therapy and for treatment provided outside Victoria were not available. VAED data for the year preceding the prostate cancer diagnosis and up to 30 days after diagnosis were assessed to identify comorbid conditions other than cancer according to the Charlson Comorbidity Index, categorised as 0 or at least 1. Age at diagnosis was categorised as under 60, 60–69, 70–79, or 80 years or more. Prostate tumours were classified by International Society of Urological Pathology (ISUP) grade,13 based solely on the Gleason score (grade 1, 6 or less; grade 2, 3+4; grade 3, 4+3; grade 4, 8; grade 5, 9 or 10) in the biopsy pathology report. The VCR, VAED and VRMDS do not capture data for all men on PSA levels and T category that would allow calculation of alternative risk groups. Socio‐economic status quintile was based on the Australian Bureau of Statistics Index of Relative Socio‐economic Disadvantage (IRSD) by Statistical Area 1 for residential address at diagnosis.14
Statistical analysis
Demographic data for men diagnosed in private and public health services were compared in χ2 tests (categorical variables) or general independence tests, using the R coin package15 (ordered variables). The proportions of men in public and private health services receiving radical prostatectomy and curative external beam radiation therapy was examined in multivariable logistic regression adjusted for age group at diagnosis, ISUP tumour grade, and comorbidity. We undertook subset analyses for each ISUP grade and socio‐economic quintile. An interaction of patient type (private, public) by year (continuous; modelled as linear trend) was fitted to assess whether differences between public and private health services were stable over time. Differences between private and public health services in treatments for men diagnosed before the age of 70 years and for men aged 70 years or more were also examined, adjusted for ISUP grade and comorbidity. Interaction between age and health service type was tested for each treatment type to assess whether treatment differences between private and public health services differed by patient age. All analyses were performed in R 3.6.3.
Ethics approval
The Cancer Council Victoria Human Research Ethics Committee approved the data linkage (reference, HREC #1312) and our analysis of linked data to study patterns of care (reference, HREC #1412).
Results
We included 29 325 Victorian men diagnosed with localised prostate cancer during 2011–2017 (Box 1). Matching biopsy‐related admissions within three months of diagnosis were available for 24 774 men (84.5%); 4551 (15.5%) were not admitted to Victorian health services for biopsy. Compared with men diagnosed in public health services, the distributions of age and ISUP tumour classification for patients diagnosed in private health services were shifted slightly to lower levels compared with men diagnosed in public services; a smaller proportion had comorbid conditions (3% v 6%), and the proportions with addresses in areas in the top two socio‐economic status categories (56% v 25%) or in major cities (80% v 61%) were larger (Box 2).
In the year following the diagnosis of prostate cancer, 10 669 men (36.4%) had radical prostatectomies (with or without associated curative radiation therapy), 3636 (12.4%) received curative external beam radiation therapy only, and 15 020 (51.2%) received neither treatment. After adjusting for age, comorbidity, and ISUP grade, radical treatment (radical prostatectomy or curative external beam radiation therapy) was more frequent for men diagnosed in private health services than for those diagnosed in public health services (odds ratio [OR], 1.40; 95% confidence interval [CI], 1.31–1.49).
Radical prostatectomy
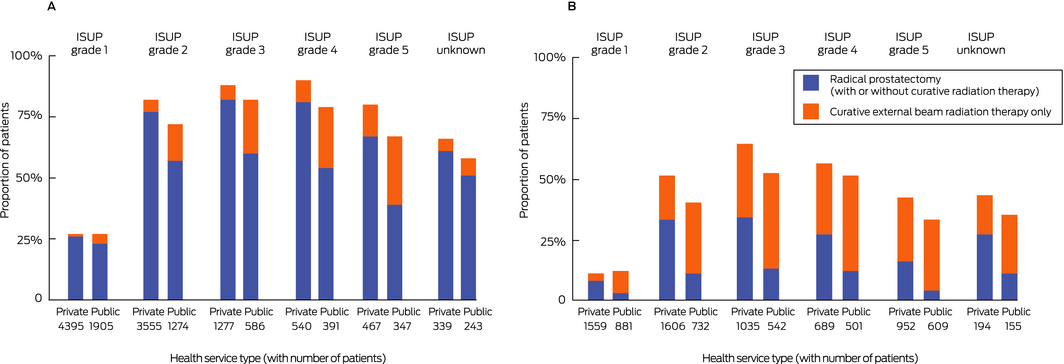
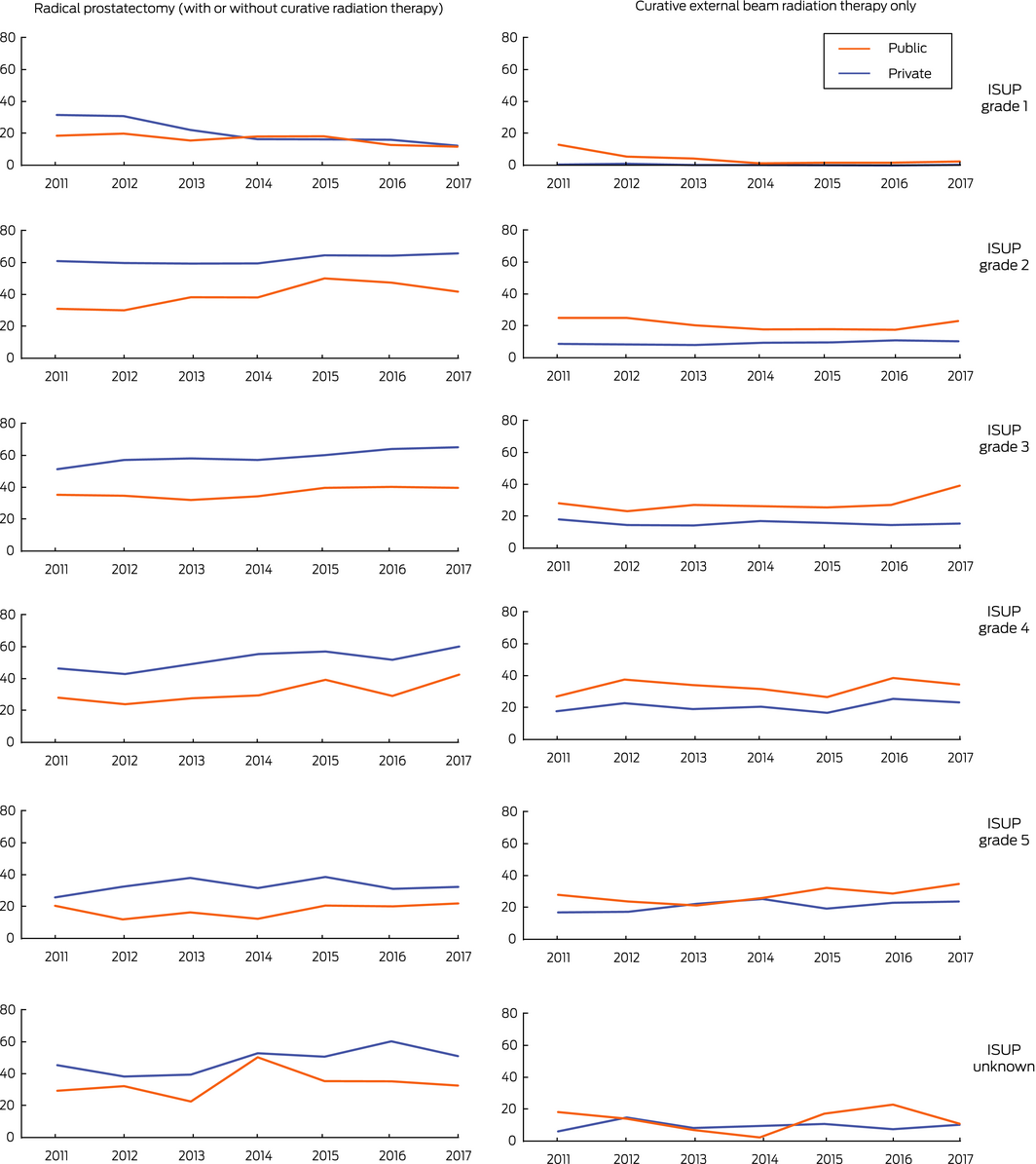
Radical prostatectomy was more frequent for men diagnosed in private health services (7263 of 16 608, 43.7%) than for those diagnosed in public health services (2260 of 8166, 27.7%). After adjusting for age, comorbidity, and ISUP grade, men diagnosed in private health services more frequently underwent radical prostatectomy (OR, 2.28; 95% CI, 2.13–2.44). Radical prostatectomy was more frequent for men diagnosed in private health services for each ISUP grade subset (Box 3; Supporting Information, table 2) and socio‐economic quintile (Box 4). The difference was greater for men diagnosed after the age of 70 years (private v public: OR, 3.45; 95% CI, 2.99–3.97) than for younger men (OR, 1.96; 95% CI, 1.81–2.12; interaction of clinic type and age: P < 0.001) (Box 5). The difference in the proportions of men treated in private and public health services who underwent prostatectomy declined between 2011 and 2017 for men with ISUP grade 1 tumours, but not for those with higher class tumours (Box 6).
Curative external beam radiation therapy
Curative external beam radiation therapy alone was less frequent for men diagnosed in private health services (1542 of 16 608, 9.3%) than for those diagnosed in public health services (1524 of 8166, 18.7%). After adjusting for age, comorbidity, and ISUP grade, men diagnosed in private health services received curative radiation therapy less often (OR, 0.45; 95% CI, 0.42–0.49). Curative external beam radiation therapy alone was less frequent for men diagnosed in private health services for each ISUP grade (Box 3; Supporting Information, table 2) and socio‐economic quintile (Box 4). The difference was greater for men diagnosed before the age of 70 years (private v public: OR, 0.29; 95% CI, 0.26–0.34) than for older men (OR, 0.60; 95% CI, 0.54–0.67; interaction of clinic type and age: P < 0.001) (Box 5). The difference in the proportions of men treated in private and public health services who underwent curative external beam radiation therapy declined between 2011 and 2017 for men with ISUP grade 1 tumours, but not for those with higher class tumours (Box 6).
Men without linked Victorian biopsy data
A considerable number of men with diagnoses of localised prostate cancer (4551, 15.5% of eligible patients) could not be classified as using a private or public health service because their biopsy dates did not match admissions to Victorian health services (Supporting Information, tables 2 and 3). Categorising all these men as either public or private patients did not markedly alter our results with respect to differences between men attending private and public health care services (Supporting Information, table 4).
Discussion
The type of care received by men with prostate cancer in Victoria during 2011–2017, after adjusting for age, tumour classification and comorbidity, differed markedly between the public and private health service systems. A larger proportion of men with localised prostate cancer diagnosed in private health services during 2011–2017 underwent radical prostatectomy (with or without subsequent curative radiation therapy) than men diagnosed in public health services (44% v 28%); a smaller proportion received curative external beam radiation therapy alone (9% v 19%). The differences between private and public health services remained substantial after assigning all patients without matched biopsy records (15.5% of patients) to the private service group (prostatectomy: 40% v 28%; curative radiation therapy: 10% v 19%). The proportion of patients undergoing surgery was greater for men diagnosed before 70 years of age and diagnosed in private health services, while the proportion receiving curative radiation therapy alone was greater for those aged 70 years or more and diagnosed in public health services.
The large randomised controlled ProTect trial found no differences in major outcomes following radical prostatectomy or curative radiation therapy alone. Prostate cancer death rates at 10‐year follow‐up were low in men diagnosed before the age of 70 years,3 with no major differences between active treatments. Further, surgery and radiation therapy each have serious adverse effects in many men. The ProtecT study16 found that urinary incontinence was more marked following prostatectomy than radiotherapy or active surveillance group at all time points examined over six years. In all treatment groups, erectile function was particularly impaired during the six months following treatment, and was most marked in those who underwent surgery. The proportions of men reporting faecal incontinence and loose stools was similar for all treatment groups, but the proportion reporting bloody stools was higher from year two for the radiotherapy group.16 It would therefore seem that factors other than high level evidence regarding control of prostate cancer and the likelihood of adverse effects, prospectively assessed in clinical trials with extended follow‐up, influence the treatment of Victorian men with localised prostate cancer.
Treatment of prostate cancer in Victoria has been described previously on the basis of data from the Prostate Cancer Outcome Registry (PCOR). In an analysis based on National Comprehensive Cancer Network risk categories,17 it was found that men diagnosed in private health services during 2008–2011 were less likely to receive surgery or radiation therapy than public patients, but the study was restricted to the 17 health services (eleven public, six private) that reported to the PCOR in 2013. A more recent analysis18 found that 28.7% of public and 50.2% of private patients underwent radical prostatectomy, similar to our findings; radiation therapy in public and private health services was not compared. Although the number of health services reporting to the PCOR has increased, the latter study included only half of all newly diagnosed cases of prostate cancer in Victoria. We analysed population‐wide VCR data linked to Victorian administrative data, including every patient diagnosed with prostate cancer in Victoria during 2011–2017, for 84.5% of whom matching biopsy admissions data were also available.
Limitations
Administrative health data are primarily collected for the purpose of reimbursing the costs of health service activity, and require translation of discharge summaries into ICD‐10‐AM codes by hospital health information managers. VAED data support the reimbursement of public health service expenses; private hospitals provide activity data to the VAED as a condition of registration, but only public health services data in the VAED are routinely audited. VAED data on private hospitals were not matched with Medicare claims data as they were not available for this study. A recent comparison of Victorian administrative data with clinical data for colorectal cancer patients at a single health service found 90% accuracy in capturing data on colorectal cancer resections.19 Administrative data often lack clinical detail. PSA levels and clinical T stage were not available for all patients, preventing classification with alternative risk models, such as National Comprehensive Cancer Network risk categories. The available administrative datasets do not include information on hormone therapy. Data on brachytherapy were incomplete and therefore excluded; it is not a common treatment, particularly in more recent years.8
Matching data on biopsy admissions were not available for 15.5% of men registered as having been diagnosed with prostate cancer. This may have been because a biopsy was not performed, but it is more likely that a biopsy was performed in an outpatient setting, such as the private rooms of a urologist. Our data were limited to the health service level.
Conclusion
Treatment of people with cancer should be consistent, safe, of high quality and evidence‐based, as described in the Cancer Council optimal care pathway for men with prostate cancer.20 Our findings indicate a notable difference between the Victorian public and private health service sectors in the treatment chosen for men with localised prostate cancer. Men with prostate cancer who have no comorbid conditions, live in areas of higher socio‐economic status, and have less aggressive disease more frequently receive their biopsy diagnoses at private health services. Further, after adjusting for the influence of these factors on their subsequent treatment, differences between choices in the private and public systems remained evident, suggesting that other factors have a strong influence on whether men undergo surgery or receive radiation therapy.
Box 2 – Demographic data for patients, by health service type for initial prostate biopsy
|
Characteristic |
Health service type |
|
|||||||||||||
|
Public |
Private |
P |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
8166 |
16 608 |
|
||||||||||||
|
Age at diagnosis (years) |
|
|
< 0.001 |
||||||||||||
|
< 60 |
1499 (18%) |
3579 (22%) |
|
||||||||||||
|
60–69 |
3247 (40%) |
6994 (42%) |
|||||||||||||
|
70–79 |
2553 (31%) |
4468 (27%) |
|||||||||||||
|
80 or more |
867 (11%) |
1567 (9%) |
|||||||||||||
|
Tumour classification (ISUP grade) |
< 0.001* |
||||||||||||||
|
1 |
2786 (34%) |
5954 (36%) |
|
||||||||||||
|
2 |
2006 (25%) |
5161 (31%) |
|||||||||||||
|
3 |
1128 (14%) |
2312 (14%) |
|||||||||||||
|
4 |
892 (11%) |
1229 (7%) |
|||||||||||||
|
5 |
956 (12%) |
1419 (9%) |
|||||||||||||
|
Unknown |
398 (5%) |
533 (3%) |
|||||||||||||
|
Charlson Comorbidity Index (excluding cancer), VAED‐derived |
|
< 0.001 |
|||||||||||||
|
None |
7674 (94%) |
16 157 (97%) |
|
||||||||||||
|
One or more |
492 (6%) |
451 (3%) |
|||||||||||||
|
Socio‐economic status (quintile)† |
< 0.001 |
||||||||||||||
|
1 (most disadvantaged) |
2416 (30%) |
1656 (10%) |
|
||||||||||||
|
2 |
1994 (25%) |
2493 (15%) |
|||||||||||||
|
3 |
1690 (21%) |
3085 (19%) |
|||||||||||||
|
4 |
1232 (15%) |
4009 (24%) |
|||||||||||||
|
5 (least disadvantaged) |
797 (10%) |
5273 (32%) |
|||||||||||||
|
Missing data |
37 |
92 |
|||||||||||||
|
Area of residence |
|
|
< 0.001 |
||||||||||||
|
Major cities |
4941 (61%) |
13 215 (80%) |
|
||||||||||||
|
Inner regional |
2516 (31%) |
2840 (17%) |
|||||||||||||
|
Outer regional/remote |
709 (9%) |
552 (3%) |
|||||||||||||
|
Missing data |
0 |
1 |
|||||||||||||
|
|
|||||||||||||||
|
ISUP = International Society of Urological Pathology; VAED = Victorian Admitted Episode Dataset. * Cases with unknown grade excluded. † Index of Relative Socio‐economic Disadvantage (IRSD) by Statistical Area 1 for residential address at diagnosis. |
|||||||||||||||
Box 3 – Treatment of men diagnosed with localised prostate cancer during the 12 months following diagnosis, 2011–2017, by International Society of Urological Pathology (ISUP) tumour grade and health service type
|
Treatment |
Private health service |
Public health service |
Adjusted odds ratio* (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Radical prostatectomy (with or without curative radiation therapy) |
|||||||||||||||
|
All ISUP grades |
7263/16 608 (43.7%) |
2260/8166 (27.7%) |
2.28 (2.13–2.44) |
||||||||||||
|
1 |
1261/5954 (21.2%) |
457/2786 (16.4%) |
1.27 (1.12–1.43) |
||||||||||||
|
2 |
3255/5161 (63.1%) |
811/2006 (40.4%) |
2.72 (2.42–3.07) |
||||||||||||
|
3 |
1402/2312 (60.6%) |
418/1128 (37.1%) |
3.41 (2.87–4.06) |
||||||||||||
|
4 |
622/1229 (50.6%) |
272/892 (30.5%) |
3.55 (2.85–4.42) |
||||||||||||
|
5 |
462/1419 (32.6%) |
162/956 (16.9%) |
3.57 (2.81–4.53) |
||||||||||||
|
Unknown |
261/533 (49.0%) |
140/398 (35.2%) |
1.80 (1.35–2.39) |
||||||||||||
|
Curative external beam radiation therapy only |
|||||||||||||||
|
All ISUP grades |
1542/16 608 (9.3%) |
1524/8166 (18.7%) |
0.45 (0.42–0.49) |
||||||||||||
|
1 |
97/5954 (1.6%) |
161/2786 (5.8%) |
0.28 (0.22–0.36) |
||||||||||||
|
2 |
447/5161 (8.7%) |
401/2006 (20.0%) |
0.38 (0.32–0.44) |
||||||||||||
|
3 |
390/2312 (16.9%) |
343/1128 (30.4%) |
0.45 (0.38–0.54) |
||||||||||||
|
4 |
248/1229 (20.2%) |
290/892 (32.5%) |
0.54 (0.44–0.66) |
||||||||||||
|
5 |
312/1419 (22.0%) |
274/956 (28.7%) |
0.69 (0.57–0.84) |
||||||||||||
|
Unknown |
48/533 (9.0%) |
55/398 (14%) |
0.61 (0.40–0.93) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; ISUP = International Society of Urological Pathology. * Adjusted for age and Victorian Admitted Episode Dataset‐derived Charlson Comorbidity Index score. |
|||||||||||||||
Box 4 – Treatment of men diagnosed with localised prostate cancer during the 12 months following diagnosis, 2011–2017, by socio‐economic status of residential address and health service type
|
Treatment, by socio‐economic status quintile* |
Private health service |
Public health service |
Adjusted odds ratio† (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Radical prostatectomy (with or without curative radiation therapy) |
|||||||||||||||
|
All patients‡ |
7263/16 608 (43.7%) |
2260/8166 (27.7%) |
2.28 (2.13–2.44) |
||||||||||||
|
1 (most disadvantaged) |
578/1656 (34.9%) |
545/2416 (22.6%) |
2.17 (1.84–2.55) |
||||||||||||
|
2 |
1017/2493 (40.8%) |
539/1994 (27.0%) |
2.06 (1.77–2.39) |
||||||||||||
|
3 |
1343/3085 (43.5%) |
497/1690 (29.4%) |
2.21 (1.90–2.57) |
||||||||||||
|
4 |
1803/4009 (45.0%) |
402/1232 (32.6%) |
1.91 (1.63–2.23) |
||||||||||||
|
5 (least disadvantaged) |
2490/5273 (47.5%) |
263/797 (33.0%) |
2.18 (1.82–2.62) |
||||||||||||
|
Curative external beam radiation therapy only |
|||||||||||||||
|
All patients‡ |
1542/16 608 (9.3%) |
1524/8166 (18.7%) |
0.45 (0.42–0.49) |
||||||||||||
|
1 (most disadvantaged) |
211/1656 (12.7%) |
502/2416 (20.8%) |
0.54 (0.45–0.65) |
||||||||||||
|
2 |
287/2493 (11.5%) |
374/1994 (18.8%) |
0.58 (0.49–0.70) |
||||||||||||
|
3 |
304/3085 (9.9%) |
303/1690 (17.9%) |
0.50 (0.41–0.60) |
||||||||||||
|
4 |
359/4009 (9.0%) |
210/1232 (17.0%) |
0.49 (0.40–0.60) |
||||||||||||
|
5 (least disadvantaged) |
371/5273 (7.0%) |
134/797 (16.8%) |
0.35 (0.27–0.44) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Australian Bureau of Statistics Index of Relative Socio‐economic Disadvantage by Statistical Areas 1 for residential address at diagnosis. † Adjusted for age, VAED‐derived Charlson Comorbidity Index and International Society of Urological Pathology (ISUP) grade. ‡ Socio‐economic status data were not available for 37 public and 92 private patients. |
|||||||||||||||
Received 18 October 2019, accepted 12 March 2020





Abstract
Objective: To compare treatments for localised prostate cancer for men diagnosed in private and public health services in Victoria.
Design: Retrospective analysis of Victorian Cancer Registry data linked to population‐based administrative health datasets.
Setting, participants: 29 325 Victorian men diagnosed with prostate cancer during 2011–2017.
Main outcome measures: Proportions of men in private and public health services receiving radical prostatectomy (with or without curative radiation therapy) or curative external beam radiation therapy alone within 12 months of diagnosis.
Results: After adjusting for age, tumour classification and comorbidity, men diagnosed in private health services received radical treatment more frequently than men diagnosed in public health services (odds ratio [OR], 1.40; 95% confidence interval [CI], 1.31–1.49). The proportion of private patients who underwent radical prostatectomy was larger than that for public patients (44% v 28%; OR, 2.28; 95% CI, 2.13–2.44) and the proportion of private patients who received curative external beam radiation therapy alone (excluding brachytherapy) was smaller (9% v 19%; OR, 0.45; 95% CI, 0.42–0.49). These differences were apparent for all International Society of Urological Pathology (ISUP) tumour grades. The magnitude of the difference for prostatectomy was greater for men aged 70 years or more; for radiation therapy alone, it was larger for those diagnosed before age 70. The differences between private and public services narrowed during 2011–2017 for men with ISUP grade 1 disease, but not ISUP grade 2–5 tumours.
Conclusion: Prostate cancer treatment choices differ substantially between men diagnosed in private and public health services in Victoria. These differences are not explained by disease severity or comorbidity.