Iron deficiency anaemia, which affects 14–22% of women of reproductive age,1 has adverse effects on pregnant women and their infants. Oral iron supplementation is the first‐line treatment, but intravenous iron therapy is sometimes preferred because of gastrointestinal effects, low patient adherence, and the delayed effect of oral iron therapy. Further, guidelines now recommend intravenous iron therapy in certain situations,2 and more rapidly infusible intravenous iron preparations have recently become available in Australia.
We investigated the use of intravenous iron by women of reproductive age, analysing dispensing data for a 10% random sample of Australians eligible to receive subsidised medicines under the Pharmaceutical Benefits Scheme (PBS).3 We included data for all women aged 18–44 years with a dispensing claim for intravenous iron during January 2013 to December 2017. Three preparations were available: iron polymaltose and iron sucrose during 2013–2017, and ferric carboxymaltose from June 2014. We calculated the annual number and rate of intravenous iron dispensing claims and iron preparation types by age group, using Australian Bureau of Statistics 2017 population data,4 and estimated overall dispensing rates by extrapolating these numbers to the national level (Supporting Information). The study was approved by the New South Wales Population and Health Services Research Ethics Committee (reference, 2013/11/494) and the federal Department of Human Services External Request Evaluation Committee.
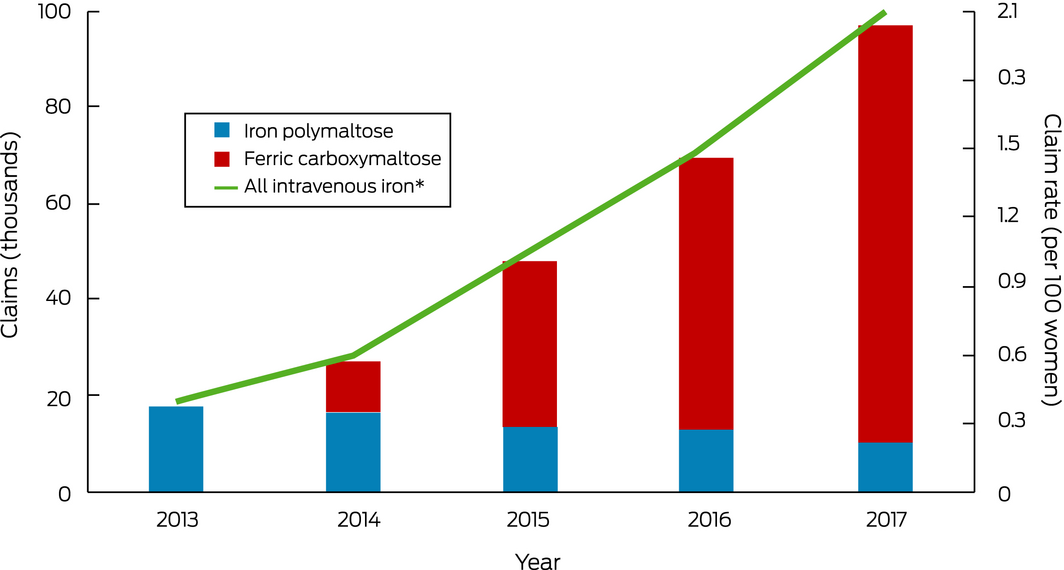
An estimated 259 700 intravenous iron dispensing claims were made for 190 490 women of reproductive age during 2013–2017; the annual number of dispensing claims increased from 17 920 in 2013 to 97 040 in 2017, and the annual rate of intravenous iron dispensing rose from 0.4 per 100 women in 2013 to 2.1 claims per 100 women in 2017 (Box). By iron type, 187 800 dispensing claims were for ferric carboxymaltose (72.3%), 71 110 for iron polymaltose (27.4%), and 790 for iron sucrose (0.3%). Most preparations were prescribed by general practitioners (111 870 claims, 43%), specialists (54 640 claims, 21%), and other medical practitioners (50 868 claims, 20%). The number of dispensing claims increased with age (18–24 years, 1.6 per 100 women; 35–44 years, 2.5 per 100 women).
In 2017, intravenous iron was dispensed to one in fifty Australian women of reproductive age, five times the proportion in 2013; in 2017, 90% of these women received ferric carboxymaltose. The optimal rate of intravenous iron treatment is unknown, and there are no comparable overseas data. As possible adverse outcomes include permanent skin staining and the risk (albeit rare) of potentially fatal anaphylaxis,5 intravenous iron should be administered in settings where allergic reactions can be treated promptly, but whether this is generally the case is not known. Intravenous iron therapy for women of reproductive age also has considerable financial implications: based on average PBS prices,6 its total annual cost increased 35‐fold, from $0.75 million in 2013 to $26.9 million in 2017. However, we have probably underestimated the use of intravenous iron therapy, as we included only PBS‐subsidised dispensing, which may not include preparations administered to public hospital inpatients.
The reasons for the rise in the use of intravenous iron are unclear, but may include increased awareness of patient blood management guidelines, the ease of treatment, and the perception that its side effect profile is more favourable than for oral iron therapy. The rapid growth raises concerns about whether it is being employed appropriately and cost‐effectively, given the potential harms and the lack of strong evidence for its value for improving quality of life and reproductive health outcomes.
Received 19 October 2019, accepted 20 January 2020
- 1. Stevens GA, Finucane MM, De‐Regil LM, et al; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: a systematic analysis of population‐representative data. Lancet Glob. Health 2013; 1: e16–e25.
- 2. National Blood Authority. Patient blood management guidelines: module 5. Obstetrics and maternity. Canberra: NBA, 2015. https://www.blood.gov.au/system/files/documents/20180426-Module5-WEB.pdf (viewed Apr 2020).
- 3. Mellish L, Karanges EA, Litchfield MJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634.
- 4. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, September quarter 2017. Mar 2018. http://www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3101.0Main+Features1Sep%202017?OpenDocument (viewed Apr 2020).
- 5. Rognoni C, Venturini S, Meregaglia M, et al. Efficacy and safety of ferric carboxymaltose and other formulations in iron‐deficient patients: a systematic review and network meta‐analysis of randomised controlled trials. Clin Drug Investig 2016; 36: 177–194.
- 6. Australian Department of Health. Pharmaceutical Benefits Scheme and Repatriation Pharmaceutical Benefits Scheme Section 85 date of supply data. Updated Apr 2020. http://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop (viewed Apr 2020).





This investigation is funded by a National Health and Medical Research Council (NHMRC) Centre of Research Excellence grant (1060407). Amanda Henry is supported by an NHMRC Early Career Fellowship (APP 1141570).
Amanda Henry, Antonia Shand, Luke Grzeskowiak and Giselle Kidson‐Gerber have received funding from the National Blood Authority for a randomised trial comparing intravenous and oral iron treatment of pregnant women.