The known: Guidelines for infant feeding and allergy prevention recommend that common food allergens be introduced by 12 months of age.
The new: An SMS/smartphone‐based program found that 86% of infants had eaten peanut by 12 months; peanut‐related reactions were reported for 2.6% of exposed children (including 1.6% with allergy‐like symptoms). Reaction rates were highest for dairy foods (8.6% of exposed infants, including 5.6% with allergy‐like symptoms).
The implications: SmartStartAllergy is a novel tool for promoting and monitoring community uptake of the ASCIA guidelines for infant feeding and allergy prevention and parent‐reported food allergy during the first year of life.
The prevalence of early childhood food allergy in Australia is among the highest in the world.1 To reduce the incidence of food allergies, the Australasian Society of Clinical Immunology and Allergy (ASCIA) recently revised their guidelines for infant feeding and allergy prevention, recommending that common food allergens, including peanut and egg, be introduced during the first year of life.2 These guidelines, underpinned by evidence from randomised controlled trials,3,4 contrast with earlier recommendations, including advice by the National Health and Medical Research Council,5 to avoid some allergenic foods during early childhood.
As introducing peanut before 12 months of age significantly reduces the risk of peanut allergy,3 early introduction should be encouraged and monitored. The most recently published data on the timing of allergenic solid food introduction and food allergy for a large population‐based cohort of infants were for 12‐month‐old children in Melbourne and surrounds born during 2007–2011; consistent with advice during the 1990s and early 2000s to avoid feeding young children allergenic foods,6 about 70% of infants in this study had not eaten peanut.7
It has been hypothesised that routinely introducing common food allergens during the first year of life could reduce food allergy rates in Australia.8 To evaluate community adoption of the ASCIA guidelines and to actively monitor changes in the incidence of allergic reactions in infants, accurate data are needed.
We therefore developed SmartStartAllergy as an active surveillance tool for promoting the ASCIA guidelines and monitoring parent‐reported allergic reactions to food during the first year of life. In this article we describe a novel approach to assessing the introduction of peanut and other common food allergens and the rates of parent‐reported food‐related allergic reactions in 12‐month‐old infants.
Methods
SmartStartAllergy is a smartphone application that incorporates the experience and infrastructure of SmartVax (www.smartvax.com.au), a program that uses short message service (SMS) and smartphone technology to actively monitor vaccine safety in real time.9 In our pilot study, we employed SmartStartAllergy, integrated with general practice management software, for two purposes:
- to determine the proportions of infants who had been introduced to peanut and other common food allergens by 12 months of age; and
- to collect information about parent‐reported food‐related allergic reactions.
We specifically focused on peanut as a potentially allergenic food type because the risk of peanut‐related allergic reactions is of substantial concern for some parents.
Recruitment of participants
The 282 general practices currently employing SmartVax (of about 7000 general practices in Australia) were invited to participate in our SmartStartAllergy study. Practices were progressively enrolled from September 2018; by May 2019, 69 general practices (24%) were participating: 30 of 81 in Western Australia, 16 of 65 in New South Wales, 12 of 68 in Queensland, three of 28 in Victoria, three of 12 in South Australia, three of 11 in the Australian Capital Territory, one of five in the Northern Territory, and one of 12 in Tasmania. Of the 69 participating practices, 43 were in major cities, 21 in inner regional areas, three in outer regional areas, and two in remote locations, according to the Accessibility/Remoteness Index of Australia (ARIA) classification.10 The Socio‐Economic Indexes for Areas (SEIFA) Index of Relative Socio‐Economic Advantage and Disadvantage (IRSAD)11 was determined for each practice postcode; 34 were in the lower five deciles, 35 in the higher five deciles. Parents of 12‐month‐old infants who attended the participating general practices were recruited directly via the SmartStartAllergy app. When their child reached 12 months of age, parents received a series of up to three automatically generated SMS messages; they could opt out of further communications by replying “STOP” to any message.
SMS protocol and questionnaire
SmartStartAllergy comprises two integrated components for collecting data about food introduction and allergic reactions. In the first component, parents receive an SMS message when their child reached 12 months of age in which they are asked, “Has [child's name] eaten foods with peanut?”; after responding “yes” or “no”, they receive a second SMS message with the question, “Has [child's name] ever had an allergic reaction to any food?”
The second component is a questionnaire accessed via a link in a third SMS message sent to participants who responded to the first two SMS questions. This questionnaire requests additional information about foods the child has eaten, foods that have caused allergic reactions, and risk factors for food allergy (including personal history of eczema and family history of allergic disease12,13) (Supporting Information, figure 1).
Parent‐reported allergic reactions to food
The first section of the questionnaire asks about parent‐reported allergies to individual foods with the question, “Which food(s) have caused an allergic reaction?”, followed by a list of common food allergens (responses: yes or no).
A second, dynamic section of the questionnaire (ie, presented only for foods for which the response to the first question was “yes”) collects details about the timing and nature of symptoms associated with the specific food. Reactions were deemed suggestive of IgE‐mediated reaction (allergy) if symptoms commenced within two hours of consumption, but instances of isolated perioral rash were excluded.
Reporting and analysis
SMS and questionnaire responses received from parents of 12‐month‐old infants between 21 September 2018 and 3 May 2019 were included in our analysis. SMS and questionnaire data are collated by SmartStartAllergy, installed locally at each general practice. The application sends the three SMS messages and receives the SMS and questionnaire responses. De‐identified data is uploaded daily to a secure central SmartStartAllergy server; data that identify participants are not accessible outside the general practice of the parent. The infant's general practitioner is automatically notified of major allergic reactions via their practice management software inbox, facilitating recall, follow‐up, or referral of the child for specialist advice. We extracted de‐identified data from the central SmartStartAllergy server for analysis.
Statistical analysis was performed in MedCalc 16.4.3 (https://www.medcalc.org). All data, including data from partially completed questionnaires, were included in analyses. Descriptive statistics are presented with 95% confidence intervals (CIs) calculated with the Clopper–Pearson exact method. Proportions were compared in χ2 tests.
Ethics approval
This investigation was approved by the University of Western Australia Human Research Ethics Committee (reference, RA/4/20/4580).
Results
The first SMS question (introduction of peanut), was sent to 3374 parents of infants (boys, 1782 [53%], girls 1572 [47%]; sex not recorded, 20); as responses were received from 1994 parents (59%), including 54 (1.6%) who opted out of the study, 1940 participants were included in our analysis (57% response rate). A total of 1831 of these parents responded to the second SMS and received the third SMS (questionnaire link), of whom 836 (46%) completed the questionnaire in full (cumulative response rate, 25%) (Supporting Information, figure 2).
The proportion of participants who commenced the questionnaire was greater for parents who had reported that their child had had a food‐related allergic reaction (SMS question 2) than for those who had not (142 of 235 [60%] v 717 of 1596 [45%]; P < 0.001).
Introduction of peanut and other food allergens
In response to the first SMS question, 1673 of 1940 parents (86.2%; 95% CI, 84.6–87.7%) indicated that their infants had eaten peanut by 12 months of age. For the three states with the highest numbers of respondents, the proportions were similar: WA, 999 of 1155 (86.5%); Queensland, 432 of 519 (83.2%); NSW, 106 of 124 (85.5%); the figure was 136 of 142 (95.8%) in other jurisdictions.
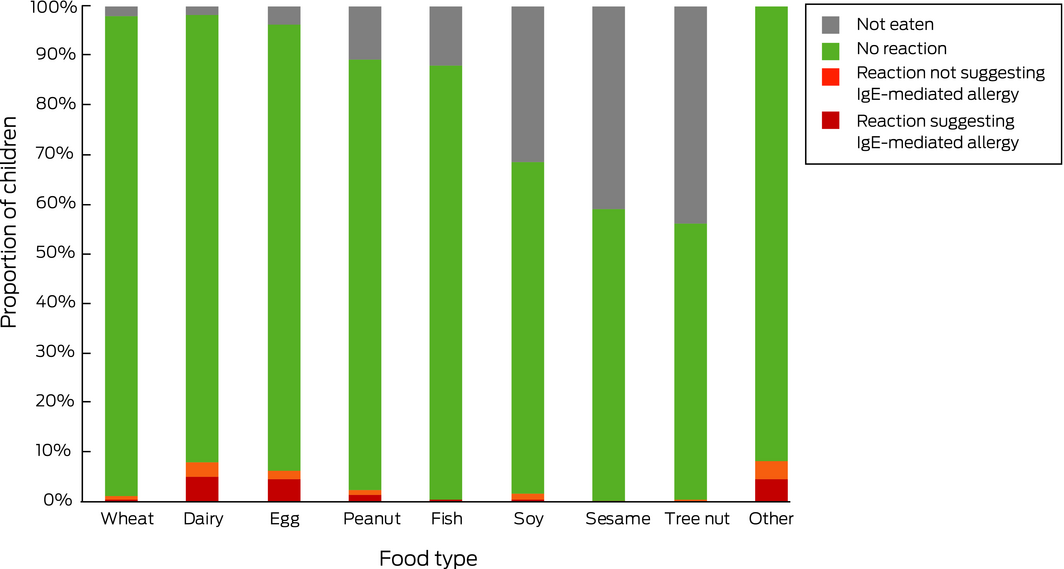
In response to the questionnaire, 767 of 857 parents (89.5%; 95% CI, 87.3–91.4%) indicated that their infants had eaten peanut by 12 months of age. This proportion was significantly greater than for the SMS responses (P = 0.017). The proportions of infants exposed to other common food allergens, as reported by questionnaire respondents, is summarised in Box 1.
Of the 839 infants for whom the relevant question was answered by parents, 226 (26.9%) had a first degree relative reported to have a food allergy. These children were less likely to have been introduced to peanut by 12 months than infants without a family history of food allergy (183 of 226 [81.0%] v 569 of 613 [92.8%]; P < 0.001).
Parent‐reported allergic reactions to food
In response to the second SMS question, 235 of 1831 parents reported food allergies in their infants (12.8%; 95% CI, 11.3–14.5%); in response to the questionnaire, 173 of 836 (20.7% of completed questionnaires; 95% CI, 18.1–23.6%) reported a total of 250 food‐related allergic reactions (Box 1, Box 2). Most respondents reported reactions to one (119 of 173, 69%) or two foods (42 of 173, 24%); twelve participants (7%) reported reactions to three or more foods. Ninety‐seven of the 250 reactions (39%) were not associated with symptoms that suggested an IgE‐mediated reaction (Box 1).
Help was sought from a health professional at the time of a food‐related reaction in 102 cases (42% of respondents to this question; Supporting Information, figure 3). Advice was sought from GPs (45 of 102, 44%), emergency departments (22 cases), other health professionals (16 cases), Health Direct (https://www.healthdirect.gov.au) or another phone advice line (eight cases), ambulance services (seven cases), or pharmacists (four cases).
Discussion
We found that most parents (86%) reported that their infants had eaten peanut before 12 months of age, consistent with current ASCIA guidelines.2 This proportion is much higher than the estimate of 30.2% determined by an Australian population survey undertaken between 2009 and 2011,7 and follows major efforts to promote the revised ASCIA guidelines in both the medical and general media.
The proportions of parent‐reported allergic reactions in our study were similar to those reported for the large HealthNuts infant cohort14 for peanut (our study, 2.6%; HealthNuts, 2.9%) and cow's milk (our study [dairy], 8.6%; HealthNuts, 6.1%). However, self‐reports of allergic reactions overestimate the true prevalence of IgE‐mediated food allergy.15 Indeed, we estimate that 39% of parent‐reported reactions in our study were probably not IgE‐mediated reactions, but may instead have been contact reactions or delayed onset, non‐IgE‐mediated reactions. Further, as the reliability of the SmartStartAllergy questionnaire for identifying IgE‐mediated food allergy was not evaluated, SmartStartAllergy‐based estimates cannot replace diagnosis by allergen challenge for determining the incidence of IgE‐mediated food allergy in infants.
As both our investigation and the HealthNuts study provide population‐based estimates of infant feeding practices and the proportions of infants with food allergy, comparing their estimates over time would be appropriate.
SmartStartAllergy and SmartVax were both initiated in WA, and general practices in this state were consequently over‐represented in our study. We had insufficient data to examine whether this bias affects the generalisability of our findings, but the proportions of infants who had been introduced to peanut in WA, NSW and Queensland were similar. We anticipate that participation in SmartStartAllergy in other jurisdictions will increase, overcoming this source of bias in future.
SmartStartAllergy data could help guide health promotion interventions by facilitating single practice, regional, or state‐based comparisons with national trends in the introduction of common food allergens and the incidence of food allergy, supporting more targeted public health programs. SmartStartAllergy can provide health promotion messages to parents of infants yet to be exposed to peanut, such as a link to information about infant feeding and allergy prevention on the National Allergy Strategy “Nip Allergies in the Bub” website (https://preventallergies.org.au). We are now evaluating the utility of SmartStartAllergy as a research and health promotion tool, focusing on infant feeding at 6 and 9 months of age and food‐related allergic reactions during the first year of life.
At the individual level, SmartStartAllergy could prompt GPs to discuss common food allergens with families of infants yet to be introduced to these foods. Consistent with previous reports,7 we found that delayed introduction of peanut was more likely when there was a family history of food allergy. While we did not examine reasons for delaying the introduction of peanut, parents may be apprehensive if a sibling has a history of allergy.16
Limitations
The response rates in our study and our inability to determine whether relevant characteristics of responders and non‐responders differed significantly limit the interpretation of our findings. More than half the initial participants answered both SMS questions, and about one‐quarter fully completed the questionnaire. It is possible that parents with personal experience of allergy were more motivated to participate and complete the questionnaire; a significantly greater proportion of participants who reported an allergic reaction in response to the SMS questions subsequently completed the questionnaire. We relied on parents’ recall of foods introduced and allergic reactions that may have occurred several months before receiving the first SMS question.
Infants in our study were recruited from those attending general practices participating in SmartStartAllergy, a potential source of bias. However, more than 80% of children aged 12 months or less in Australia have visited general practices, a greater proportion than have used any other single category of health service.17 Recruiting infants through general practices may therefore be appropriate for obtaining a sample representative of this age group, although we have not established whether general practices participating in SmartStartAllergy are typical of all general practices.
Although the postcode‐based socio‐economic status of a general practice may not be the same as that of its patients, the distribution of IRSAD deciles in our study suggests that the participating practices were equally balanced between upper and lower socio‐economic status levels. With the anticipated expansion in the number of participating practices and the increasing number of questionnaire respondents, further evaluation of the effect of the socio‐economic status of participants will be possible.
The HealthNuts cohort was recruited from local government immunisation clinics in metropolitan Melbourne (45.6% of infants were immunised at such clinics in Victoria in 2008), and included participants from postcodes of higher mean socio‐economic status and with mothers of a higher median age than the general Victorian population.18 The limited demographic data we have for our respondents prevented us from comparing our participants with either the HealthNuts cohort or the overall Australian infant population.
Conclusions
By developing SmartStartAllergy and engaging with GPs, we have provided data that suggest a shift in infant feeding practices in Australia with respect to introducing common food allergens. We found that most infants now receive peanut during their first year of life, consistent with ASCIA guidelines.
By expanding SmartStartAllergy to more general practices across Australia, our program offers a unique opportunity for collecting and reporting large scale data on the introduction of common food allergens during the first year of life and parent‐reported food‐related reactions. Confirming IgE‐mediated allergic reactions remains important, but SmartStartAllergy may directly improve patient care by delivering targeted health promotion messages, reporting significant reactions to GPs, and facilitating the development of local, streamlined referral processes.
Box 1 – Introduction of food types, parent‐reported reactions to food, and reactions suggesting IgE‐mediated food allergy in infants (questionnaire responses)
|
Food |
Introduced by 12 months of age |
Parent‐reported reactions |
Reactions suggesting IgE‐mediated allergy |
||||||||||||
|
Number |
Proportion (95% CI) |
Number |
Proportion (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Wheat |
839/857 (98%) |
9/835 |
1% (0.5–2%) |
4/835 |
0.5% (0.1–1%) |
||||||||||
|
Dairy |
837/857 (98%) |
72/835 |
8.6% (6.8–11%) |
47/835 |
5.6% (4.2–7.4%) |
||||||||||
|
Egg |
820/857 (96%) |
56/817 |
6.9% (5.2–8.8%) |
40/817 |
4.9% (3.5–6.6%) |
||||||||||
|
Peanut |
767/857 (89%) |
20/764 |
2.6% (1.6–4.0%) |
12/764 |
1.6% (0.8–2.7%) |
||||||||||
|
Fish |
758/857 (88%) |
4/756 |
0.5% (0.1–1%) |
4/756 |
0.5% (0.1–1%) |
||||||||||
|
Soy |
580/857 (68%) |
14/580 |
2.4% (1.3–4.0%) |
4/580 |
0.7% (0.2–2%) |
||||||||||
|
Sesame |
509/857 (59%) |
2/509 |
0.4% (0–1%) |
1/509 |
0.2% (0–1%) |
||||||||||
|
Tree nut |
484/857 (56%) |
3/483 |
0.6% (0.1–2%) |
2/483 |
0.4% (0.1–2%) |
||||||||||
|
Other |
— |
70/847 |
8.3% (6.5–10%) |
39/847 |
4.6% (3.3–6.2%) |
||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. Not all participants completed all questions; table includes all available responses to each question. * Excluding isolated perioral rash. |
|||||||||||||||
Received 19 June 2019, accepted 14 October 2019






Abstract
Objectives: To estimate the proportion of infants introduced to peanut and other common food allergens by 12 months of age; to collect information about parent‐reported reactions to food.
Design, setting: Observational cohort study, applying the SmartStartAllergy SMS protocol and online questionnaire to parents of 12‐month‐old infants attending 69 Australian general practices between 21 September 2018 and 3 May 2019.
Participants: 3374 parents recruited via the 69 participating general practices.
Main outcome measures: Proportions of infants who had eaten peanut and other common food allergens; proportions with parent‐reported reactions to food.
Results: 1940 of 3374 invited parents participated in the study (response rate, 57%), of whom 836 (46%) completed the online questionnaire. At 12 months of age, 1673 of 1940 infants had eaten peanut‐including foods (86.2%; 95% confidence interval [CI], 84.6–87.7%); 235 of 1831 parents (12.8%; 95% CI, 11.3–14.5%) reported food‐related reactions. Questionnaire responses indicated that dairy was the food type most frequently reported to cause a food‐related reaction (72 of 835 exposed infants, 8.6%; 95% CI, 6.8–11%); peanut‐related reactions were reported for 20 of 764 exposed children (2.6%; 95% CI, 1.6–4.0%). 97 of 250 parent‐reported reactions to food (39%) did not include symptoms that suggested an IgE‐mediated allergic reaction.
Conclusion: Infant feeding practices in Australia have changed over the past decade; a large majority of infants are now fed peanut before 12 months of age. The SmartStartAllergy program allows monitoring of infant feeding practices in primary care, as well as of parent‐reported reactions to food in infants.