Usual care must be rapidly adapted to isolate, assess and test large numbers of patients during the COVID‐19 pandemic
Coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in China in late 2019.1 COVID‐19 is an example of a high consequence infectious disease that may present to an Australian hospital. These infections are uncommon in Australia and, in most cases, were imported from overseas. Less frequently, there is onward local transmission, such as during the influenza A(H1N1)pdm09 pandemic in 2009.
High consequence infectious diseases present unique challenges to Australian hospitals. Their rarity leads to unfamiliarity and loss of institutional knowledge between events. Many hospitals operate at near maximal capacity between outbreaks and have limited surge capacity.2 Protocols designed to manage single patients require adaptation to situations where larger numbers of patients require isolation, assessment and testing for infection.
While every Australian hospital has a mass casualty or disaster protocol, these are developed for all hazards and may not address problems specific to high consequence infectious diseases, including:
- the need to rapidly identify and isolate potentially infectious patients to prevent nosocomial transmission;
- the complexity of rapid triage and assessment on frequently evolving epidemiological and clinical grounds;
- the difficulty of differentiating high consequence infectious diseases from more common but clinically similar conditions;3
- the absence of rapid diagnostic tests to aid clinical decision making; and
- the potential for a prolonged surge for weeks to months during which time the workforce may be affected by both infection and absenteeism.
Here we describe the strategic approach of the Royal Melbourne Hospital to triage and screen patients who have presented at risk (or concerned that they are at risk) during the early phases of COVID‐19. Our resources may be of value to other organisations refining their triage and clinical algorithms.
The Royal Melbourne Hospital response
The Royal Melbourne Hospital is an adult tertiary referral centre and the designated state‐wide provider for quarantinable diseases. The emergency department (ED) treats over 80 000 patients annually.
From 6 January 2020, we instituted tools to identify at triage those patients with risk factors for COVID‐19 and rapidly isolate them. Initially, there was capacity to assess patients in one of three existing negative pressure rooms. On 25 January, the first patient with COVID‐19 in Australia, who had arrived in Melbourne on a flight from Guangzhou, was confirmed. The Victorian Department of Health and Human Services informed all passengers on the flight of their possible contact with the patient, leading to a significant surge in presentations to the Royal Melbourne Hospital.
Box 1 presents an overview of the challenges in managing high consequence infectious diseases and details of our coordinated approach. Key components that can be used by other services are detailed below.
Governance
Unlike other major incident responses, which tend to be short‐lived, response to an outbreak requires a sustained response that will inevitably have an impact on other clinical services. A governance process that includes executive sponsors and senior clinical leaders is essential. The Royal Melbourne Hospital COVID‐19 response leveraged an existing code brown (external emergency) pandemic subplan and clinical code yellow (internal infectious disease emergency) plans as a governance framework. A governance group including medical and nursing executives and senior clinicians from the ED, infectious diseases, infection prevention services and microbiology meet regularly.
A single standard operating procedure exists on our hospital intranet that provides all clinically relevant information for frontline health care workers (eg, personal protective equipment guidelines, current case definitions, patient assessment algorithms). It is updated frequently given the dynamic situation and, thus, functions as a living document for staff. This document provides 24/7 access to an authoritative source that supports junior and senior staff alike to feel confident in their practices and approach.
Infrastructure
Establishment of a fever clinic.
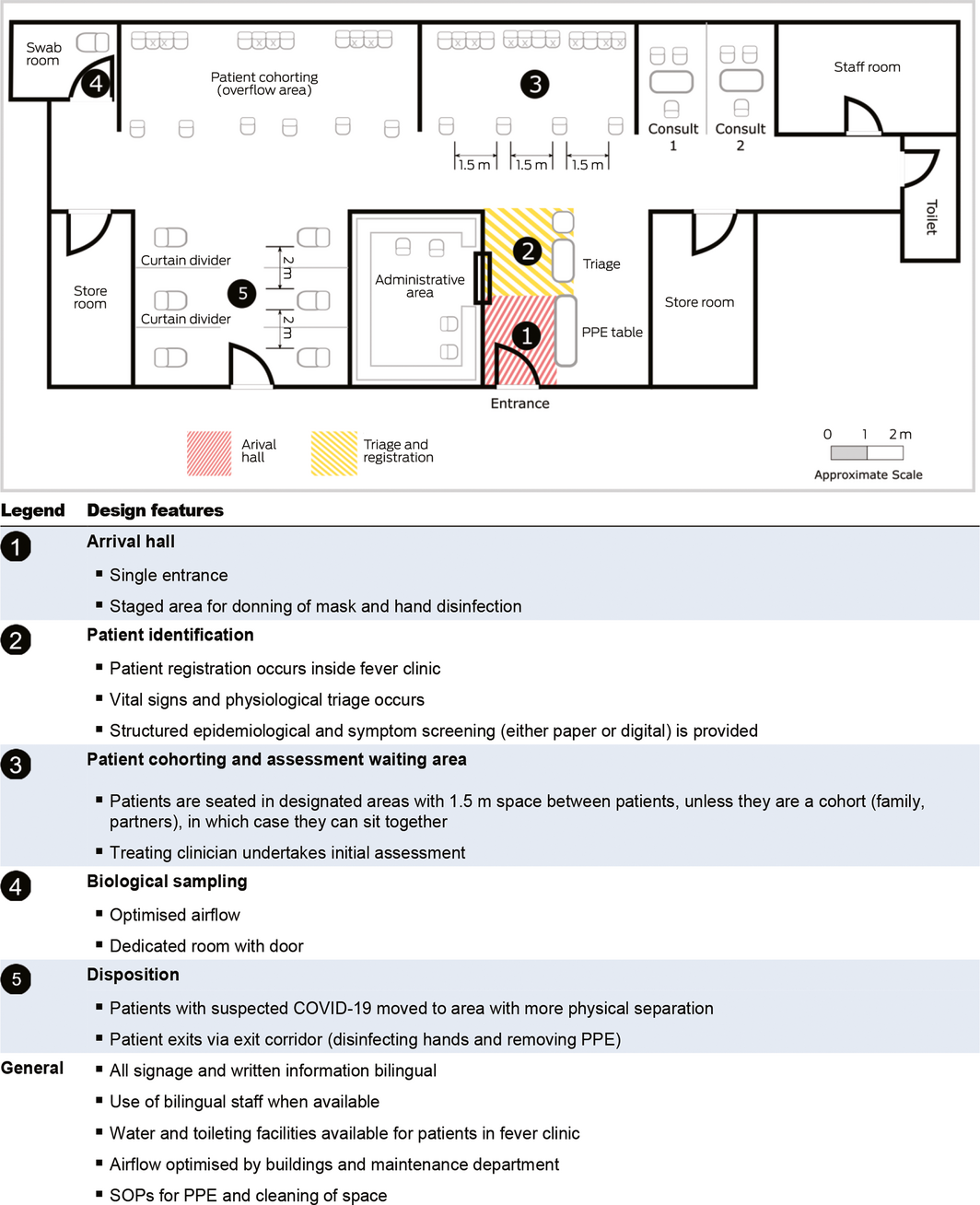
A particular design feature that may be adopted by other facilities is the rapid establishment of an out‐of‐department fever clinic. In response to the first surge of patients, we rapidly repurposed the nearby hospital transit lounge, which was closed for the weekend, into a fever clinic (Box 2). The clinic received its first patient within 2 hours of notification from the Victorian Department of Health and Human Services of the first local case. In its first 7 days, we assessed 109 patients. We discharged over 90% of patients within 4 hours of arrival. We retain this model as patient numbers continue to increase.
In this model of care, patients are physically segregated from the rest of the ED into a dedicated rapid assessment and treatment space from their arrival, limiting exposure to other patients. The main benefit of this approach is that cases yet to be identified can be an important contributor to nosocomial transmission; therefore, early separation and detection are vital.4,5 However, immediate recognition of cases is difficult due to unfamiliarity with the disease, overlap in clinical presentation with more common illnesses, and due to patient wait times.
Our fever clinic model of care was based on the success of this model in Toronto and Taiwan during the severe acute respiratory syndrome (SARS) outbreak,6 where no transmission was reported in these facilities despite hospital exposure being implicated in the majority of cases in these regions (eg, it was the presumed source of exposure for 72% of patients in Toronto7,8). It has also been reported as an effective strategy for triaging patients in Wuhan for COVID‐19.9 Similar approaches appear to have been used in other countries, but detailed descriptions are not yet available in the literature. In Australia, segregation of major incident patients was exemplified by the Royal Darwin Hospital, which functioned as the forward receiving hospital for medically evacuated patients during the 2002 Bali bombings.4
The benefits of establishing a fever clinic include:
- protecting an existing environment for the maintenance of business continuity;
- facilitating protocolised interventions for spatially clustered groups of patients;
- providing a physical location to send additional disaster resources without cluttering areas of core business; and
- enhancing record‐keeping.
Limitations of our approach include the additional staffing required, operational impact of loss of transit lounge, staff unfamiliarity with the location of resources (such as resuscitation trolleys), and a slightly further distance from resuscitation bays if patients deteriorate. Moreover, we were also concerned about the risk of stigmatisation of patients who are seen to be segregated from the main ED waiting room cohort.
Implementation of electronic self‐registration and self‐screening.
A surge related to an emerging infectious disease provided our clerks’ department with a confluence of unique administrative and logistical challenges. These included:
- a high proportion of patients came from a non‐English speaking background;
- contact tracing and follow‐up requires accurate registration and an extended suite of contact details, but usual disaster response medical records protocols generate only anonymised patient registrations;
- non‐clinical staff (ward clerks) unfamiliar with personal protective equipment would be required to extensively interview patients to confirm details at some point;
- patients came in bursts, producing delays in registration;
- manual screening paperwork and registration papers provide a potential fomite for disease transmission; and
- our ED is paper‐free under usual circumstances.
We developed a novel solution to this problem, leveraging the fact that over 91% of Australian citizens and over 96% of Chinese citizens own a smartphone5,10 and converted an initial paper‐based bilingual screening tool to an online one. This is hosted using the research electronic data capture (REDCap) tool (www.projectredcap.org).11
Patients are directed to a secure website optimised for use on a smartphone. The registration portal is free to use. They answer questions regarding their epidemiological risk (such as a detailed travel history, or being a health care worker), clinical risk factors (such as being immunocompromised) and symptoms. Results are immediately fed to remote clinical computers where ward clerks can register the patient without direct patient contact and clinicians can see screening information before their clinical encounter.
While not yet tested under a pandemic scenario, we anticipate this method of self‐registration may be particularly useful in the event of a significant surge in patient numbers. Triage sieve and sort of patients can be rapidly undertaken by clinicians who are fed real‐time registration data. Compared with usual mass casualty principles, the inclusion of epidemiological data in the electronic tool is valuable for triage in this setting to screen out the relatively high proportion of patients with perceived, but not actual epidemiological risk factors.
Our REDCap infrastructure is available in the Supporting Information for adaptation by other health services.
Conclusion
The importation of emerging infections into Australia is rare, and onward transmission is rarer still. As the Royal Melbourne Hospital received a surge in patients who required screening for COVID‐19 relatively early during the current outbreak, our recent observations may provide opportunities for other hospitals to enhance their preparedness and response plans. We prioritise prevention of nosocomial transmission (using a scalable, separated fever clinic) early planning for worsening surge (adopting scalable solutions) and clear clinical governance (providing malleable and accessible centralised resources).
Box 1 – Elements of the Royal Melbourne Hospital clinical response
|
Element of response |
Challenges |
Approach used |
|||||||||||||
|
|
|||||||||||||||
|
Clinical governance |
Multiple clinical units involved, with tangible impacts on business as usual activity and frequent changes to the model of care and the expectations |
Where possible, we operated within existing plans and policies. Daily executive and head of unit level huddles were instituted initially, and then stepped down to weekly as needed, producing hospital agreement on messaging and expectations of all teams and sharing of information between executive, infectious diseases (ID), infection prevention services (IPS) and emergency medicine (EM). COVID‐19 multidisciplinary working groups were formed within the ED and ID clinical units |
|||||||||||||
|
Infrastructure |
A space was needed to accommodate the extra patients while maintaining infectious isolation among them, and between them and the rest of the ED census |
A graduated response used with existing ED negative pressure rooms used for small numbers, a cohort subwaiting area was created when several patients were present in the ED at once, and a separate fever clinic was created in the nearby transit lounge used for surge response |
|||||||||||||
|
Infection prevention and control practices |
Transmission dynamics are incompletely understood and there is a risk of nosocomial amplification (especially during aerosolising procedures) |
Education sessions, posters, and videos used to reinforce PPE training; nebulisers removed from dedicated treatment space; hand sanitiser stations; PPE stations and infectious waste bins deployed; and a SOP employed for aerosolising procedures (Supporting Information) |
|||||||||||||
|
Clinical care (including triage, assessment and testing) |
There is rapidly evolving understanding of clinical and epidemiological characteristics of the disease. Staff lack familiarity with the disease and with the roles performed (concierge nurse, fever clinic doctor), while the normal ED and hospital functions need to continue alongside |
Creation of a SOP including clinical algorithms for triage, assessment and biological sampling as a living document hosted on the hospital intranet, and updated as needed and used as a single source of truth for clinical staff. Gradual transition to algorithm‐driven assessment by junior medical staff to free up senior staff for unwell patients. Action cards with role descriptions were provided in the SOP for all fever clinic staff |
|||||||||||||
|
Communication with patients |
Initially, most patients were Mandarin‐speaking |
Bilingual signage (English and Mandarin) deployed in the fever clinic, and bilingual patient resources and screening questionnaire generated. Discharge information sheets specific to different tiers of risk were translated into Mandarin and provided to all patients discharged from the fever clinic |
|||||||||||||
|
Human resources |
Maintenance of staff competence and confidence essential for safety and prevention of absenteeism |
Regular education sessions to provide updated clinical information and epidemiology, train in PPE, and answer questions |
|||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019; ED = emergency department; PPE = personal protective equipment; SOP = standard operating procedure. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- 1. Wuhan Municipal Health Commission. Report of clustering pneumonia of unknown etiology in Wuhan City; 2019. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 (viewed Feb 2020).
- 2. Traub M, Bradt DA, Joseph AP. The Surge Capacity for People in Emergencies (SCOPE) study in Australasian hospitals. Med J Aust 2007; 186: 394–398. https://www.mja.com.au/journal/2007/186/8/surge-capacity-people-emergencies-scope-study-australasian-hospitals.
- 3. Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 2018; 18: 217–227.
- 4. Palmer DJ, Stephens D, Fisher DA, et al. The Bali bombing: the Royal Darwin Hospital response. Med J Aust 2003; 179: 358–361. https://www.mja.com.au/system/files/issues/179_07_061003/pal10082_fm.pdf.
- 5. Deloitte China. China Mobile Consumer Survey 2018. Beijing: Deloitte China; 2018. https://www2.deloitte.com/content/dam/Deloitte/cn/Documents/technology-media-telecommunications/deloitte-cn-2018-mobile-consumer-survey-en-190121.pdf (viewed Feb 2020).
- 6. McDonald LC, Simor AE, Su IJ, et al. SARS in healthcare facilities, Toronto and Taiwan. Emerg Infect Dis 2004; 10: 777.
- 7. Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003; 169: 285–292.
- 8. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003; 289: 2801–2809.
- 9. Zhang J, Zhou L, Yang Y, et al. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med 2020; 8: e11–e12.
- 10. Deloitte Access Economics. Mobile nation 2019: the 5G future. Australian Mobile Telecommunications Association; 2019. https://www2.deloitte.com/au/en/pages/economics/articles/mobile-nation.html#2019 (accessed Feb 2020).
- 11. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) — a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381.





We thank Bruce Garbutt, Liz Orr, Naomi Laidlaw, Steve Pincus, Susan Harding, Elizabeth Bradbury, Ben Smith, Melinda Truesdale and Erhui Cai (Second Affiliated Hospital of Shantou University Medical College), who were instrumental in creating and refining the model of care and who contributed to early drafts of this article, and Jonathan Knott, who provided significant guidance on description of the model. Amanda Rojek is funded by Open Philanthropies.
No relevant disclosures.