The known: Participation rates for clinical research studies have been declining over the past four decades.
The new: Traditional print invitation mail‐outs and reminder letters with hard copy and electronic reply options, supplemented by snowball sampling and Facebook advertising, achieved an overall participation rate of only 3%, or 10% of the target population, despite the minimal study burden for prospective participants.
The implications: To achieve high participation rates, multiple modalities of recruitment, tailored to the age group of the target population, need to be employed.
Recruitment is a critical element of any research study, and its success depends on the sampling frame, appropriate methods for contact and follow‐up, and the participation rate in the target group. Sampling frames for research studies in Australia have traditionally relied on the Australian Electoral Commission roll and telephone listings.1,2,3,4 However, access to the electoral roll has become increasingly problematic, and the shift to unlisted mobile phones means that large proportions of target populations are not included in telephone directories.2,5 Some population‐based studies have used the Medicare database of the Australian Department of Human Services (DHS), as it includes recent and reliable contact information for most people in Australia.4,6 This database also has limitations that can lead to sampling bias; for example, prospective participants may be inappropriate for recruitment (terminally ill, cognitively impaired, or deceased individuals) and people may be excluded from recruitment because their details have not been updated.2
Willingness to participate in research has decreased in recent times, and recruitment is often the rate‐limiting step for randomised controlled trials (RCTs).7,8,9,10 Typical participation rates for health surveys, for example, declined around the world from 80% in the 1980s to 40–50% in 2015.7 Trials with minimally restrictive inclusion criteria achieve better response rates than those with more restrictive conditions,9 and response rates for therapeutic trials are higher than for prevention trials.10 Several systematic reviews have sought to identify methods for enhancing recruitment for RCTs,8,11,12,13,14 one identifying 137 different strategies.13 Providing monetary incentives was the most prominent strategy, and was also a good strategy for recruiting participants for cardiovascular disease prevention trials.15 However, this approach is not always successful, and is expensive for large scale clinical trials. A review of publicly funded clinical trials in the United Kingdom found that only 55% achieved their target sample sizes.8 Newer approaches, including recruitment via social media, can increase message reach and response rates.16,17,18
The Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE), a community‐based, randomised, placebo‐controlled clinical trial of the value of the 23‐valent pneumococcal polysaccharide vaccine (23vPPV) for preventing cardiovascular events,19 recruited participants in six cities. In this article, we describe the challenges of recruiting participants for this large scale Australian study and discuss potential lessons for future studies.
Methods
Sampling frame
To minimise volunteer bias, we used the Medicare database for establishing our sampling frame. The DHS randomly selected people aged 55–60 years, stratified by sex (equal numbers of men and women) and site (six locations, based on the data collection clinics). To maximise the likelihood of participation, only people with addresses within 25 km of the participating centres were included, and their data were cross‐linked with the National Death Index to avoid contacting people who had recently died.
Mailing protocol
The DHS employed a mail house to prepare the mail‐out to potential participants (Box 1). Each person received cover letters from the DHS and the investigators, an invitation letter, a screening questionnaire for assessing study eligibility, and a reply‐paid envelope. Potential participants could complete the questionnaire on paper or on the AUSPICE website (https://auspice.apps.hmri.com.au), which includes a short video on the scientific background to the study. Cover and reminder letters were posted 2 weeks after the initial mail‐out by the DHS; reminder phone calls were not made.
Screening questionnaire
To be eligible for the study, an individual was required to have at least two of three risk factors for cardiovascular disease (CVD):
- hypertension: self‐reported (based on a physician's diagnosis), or receiving one or more antihypertensive medications;
- hypercholesterolaemia: self‐reported (based on a physician's diagnosis), or receiving lipid‐lowering medication;
- overweight/obesity: body mass index exceeding 27 kg/m2 or a waist circumference exceeding 88 cm (women) or 102 cm (men).
Exclusion criteria included a history of CVD, an immediate indication for receiving or a history of having received the pneumococcal vaccine, and being over 65 years of age (as pneumococcal vaccination is part of the national immunisation schedule for people in this age group, administering a placebo would be unethical).19
Recruitment
After their questionnaire responses were entered into the database, eligible participants were notified of their eligibility by text message or email. They also received an information statement and consent form electronically (or on paper, for people without email addresses) before they were contacted to organise a clinic appointment. A reminder text message or email was sent the week before and the day before their appointment. Recruitment began in February 2016 and was completed by December 2017 at six sites: Newcastle (Hunter Medical Research Institute), Central Coast (Gosford Hospital), Canberra (Canberra Hospital), Melbourne (Caulfield Clinical Trials Centre), Adelaide (South Australian Health and Medical Research Institute), and Perth (Institute of Respiratory Health).
Expected participation rate
The proportion of eligible persons in the catchment areas was estimated to be 29% of people aged 55–60 years, based on the results of a large cohort study in Newcastle.3 We anticipated a 33% participation rate by eligible people, based on the minimal study requirements (one postal questionnaire, one clinic visit) and the potential benefits to those at high risk of CVD. The overall anticipated participation rate was thus 33% × 29%, or about 10%, and we accordingly planned to mail 60 000 invitation letters to obtain the sample size of 6000 participants required for 80% power to detect a hazard ratio of 0.82 (α = 0.05).19
Responses
Eligible participants were sent a short questionnaire on basic demographic characteristics (age, sex, self‐reported blood pressure, cholesterol and weight status). We investigated whether the characteristics of participants who responded to the first invitation (early responders) differed from those of people who responded only after receiving the reminder (late responders). We also investigated response rate according to level of socio‐economic disadvantage; postcodes of residence were allocated to Index of Relative Socio‐economic Disadvantage (IRSD) quintiles.20 We assessed the reach and impact of our recruitment strategy in Google Analytics (https://marketingplatform.google.com/about/analytics).
Statistical analyses
Participation rates are reported overall and by study site and IRSD quintile. Characteristics of early and late responders are presented as means with standard deviations (SDs) for continuous variables and frequencies and percentages for categorical variables, and compared in Student t tests and χ2 tests respectively. All statistical analyses were conducted in SAS 9.4 (SAS Institute).
Ethics approval
Approval for the clinical trial and for data linkage was granted by the Human Research Ethics Committees (HRECs) governing each trial centre: the University of Newcastle HREC (reference, H‐2014‐0064), the Hunter New England Local Health District (also for Central Coast Local Health District; references, 15/08/19/3.01; HREC/15/HNE/298), the ACT Health HREC (reference, ETH.7.14.177), the Australian National University HREC (reference, Human Ethics Protocol 2015/523), the SA Health HREC (reference, HREC/16/SAH/45), the Monash University HREC (reference, CF14/3016‐2014001638), the Department of Health WA HREC (reference, 2015/54), and the University of Western Australia HREC (reference, RA/4/1/7101).
Results
Recruitment: response rates
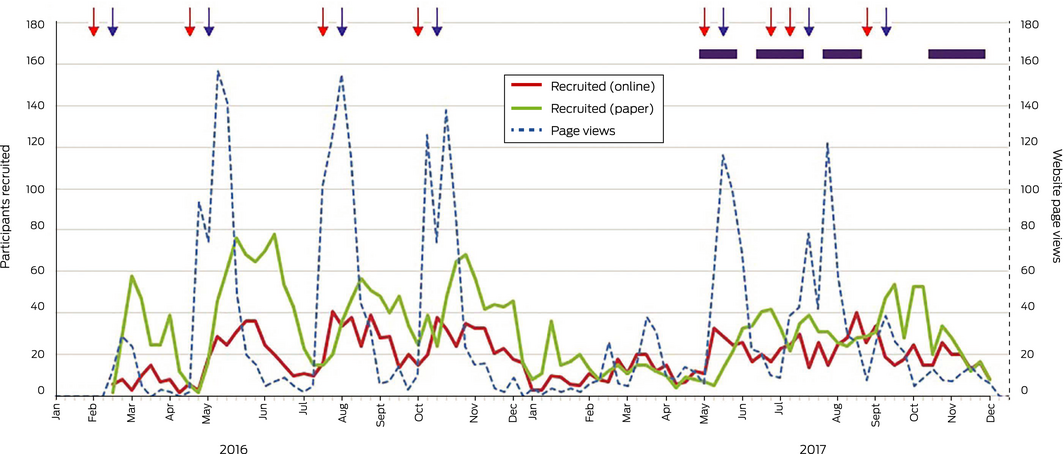
In the first of four waves of recruitment planned for February–December 2016, 2500 invitations were sent for each of the six participating centres; a total of 254 participants were recruited during this wave (1.7% participation rate). The mail‐out was therefore increased to 5000 per site per wave, and we also encouraged people who received invitations to participate by advertising in local media (radio, television, newspapers), primarily at the start of the recruitment wave. In June–July 2016, we began snowball sampling; that is, asking people who responded to invitations, regardless of whether they were eligible for the trial or not, to invite family and friends to participate.
By December 2016, 2800 participants had been recruited, or fewer than 50% of our recruitment target. The study steering committee extended recruitment for a further year and sought strategies for increasing recruitment. Two of the six sites (Central Coast, Perth) did not continue recruitment into the second year because of other commitments. The sample size calculation for the trial was reviewed; by extending the follow‐up period of our study from 4 to 6 years, we could reduce the required sample size from 6000 to 4500 participants without reducing its statistical power, after taking into account the unblinding and censoring of participants who reached age 65.
In the second year of recruitment, there were two mail‐outs with reminders for each of the four sites, and one final mail‐out for two sites (Melbourne, Newcastle). Facebook advertising commenced for all sites, targeting people in selected postcodes within 25 km of each centre. Recruitment was completed by December 2017, by which time 4725 participants had been recruited.
Recruitment: participation rates
A total of 154 992 invitation letters (and an equal number of reminders) were sent by the DHS. Overall, 21 526 invited people (14%) responded by completing the screening questionnaire (in whole or in part), while 4725 people who responded to the screening questionnaire (22%) completed clinic visits and were randomised for participation in the trial, an overall participation rate of 3% (Box 2, Box 3).
As this participation rate included many people who did not meet the CVD risk factor inclusion criteria, we also calculated the participation rate for respondents eligible for the trial. We estimated that 47% of eligible people completed screening, and that 10% attended a clinic and were randomised for participation in the trial (Box 2).
Of the 4697 participants for whom complete baseline data were available, 3951 (84%) responded to the invitation only after receiving a reminder. Early and late responders who were eligible for the trial were similar in most demographic and clinical characteristics. Equal proportions of early responders replied online or on paper, whereas 65% of late responders replied on paper (Box 4).
There were several postcodes within 25 km of each study site. The participation rate in areas of IRSD quintile 1 (greatest disadvantage) was 2.0%, whereas in quintiles 2–5 participation rates were 3.0–3.1% (Box 5).
Of the 4725 study participants who were randomised for the study, 31 (0.7%) had no email address or mobile phone number and had to be contacted by mail.
Social media
The number of page views for the AUSPICE website consistently increased sharply following mail‐outs and corresponded to peaks in invitee responses. The initial DHS mail‐out generated almost immediate increases in the numbers of page views for the AUSPICE website and of online responders. There was a lull in all activity during the 2016–17 Christmas and summer holidays. Free and paid Facebook advertisements placed at various points during 2017 to recruit people in areas close to the participating sites did not influence the numbers of either hard copy or online responses (Box 6). We did not systematically collect the source of referral (direct or snowball) for recruited participants.
Costs of recruitment
The mail‐outs of invitations with questionnaires and of reminder letters (309 984 letters and reply‐paid envelopes in total) cost $357 775, including printing and postage costs, or $75.72 per participant. Facebook advertising ($14 162.45 in year 2) and start‐up funds for five of the six clinics (5 × $44 390.40 in year 1) increased total recruitment costs to $593 890, or $125.69 per participant.
Discussion
The response rate to invitations to participate in an RCT of an established vaccine, despite the modest burden on prospective participants during screening, was about 3% overall, or an estimated 10% of the target recruitment population. Low response rates have been reported for many epidemiological studies in recent years.7,21,22,23 Proposed explanations have included lack of interest by potential participants in information about their own health and in volunteering, their preference for less intrusive and time‐demanding studies, the lack of incentives to participate, mistrust of the scientific community, the increasing volume of junk mail received by many people, and not clearly identifying the organisers of the RCT in invitations with institutional logos and academic signatures.17,21,22,23 For vaccine trials, distrust of vaccines and fear of vaccine‐induced disease may also reduce participation.24 Oversampling of target groups itself depresses the participation rate.21
We accordingly provided prospective participants with information about the trial, including a video on the science underlying the study and what was required of participants, available both on the study website and on YouTube (https://youtu.be/BScWxe3aAIE). We attempted to minimise the burden on participants, including that of data collection; the screening questionnaire took about 3 minutes to complete, the postal questionnaire 20 minutes, and the clinic visit about 45 minutes. The invitation letters included colourful institutional logos on the letterhead and information about the researchers involved in the study. We advertised on television and radio, as well as in print and social media, to encourage participation by invitees. We did not offer any financial incentives, both to reduce costs and because the evidence on their effectiveness is equivocal.12,25 However, we acknowledged that participants in the active intervention group would receive a vaccine they would normally not receive for free, under the National Immunisation Program, until age 65.
Site‐specific overall response rates ranged between 2.0% (Perth) and 4.5% (Canberra). At the Canberra site, the private rooms of a cardiologist were adapted for clinical research, providing convenient parking and a non‐hospital setting. Further, a sub‐study examining the mechanisms of any protective effects of the vaccine was also undertaken here; 1000 participants received free blood tests (for serum anti‐oxidised low‐density lipoprotein antibody), pulse wave velocity tests, electrocardiography, and carotid intima media thickness measurements at baseline and follow‐up. Despite the greater burden on participants, the sub‐study may have been regarded positively, as a free health check. It is difficult to know whether any of these factors explained the higher participation rate in Canberra, which may also have been related to the higher socio‐economic status of the catchment area, despite the general lack of influence of socio‐economic status (measured by postcode IRSD quintiles) on response rates.
More than 80% of eventual participants responded to the invitation only after receiving the reminder letter, indicating that this was a worthwhile, cost‐effective measure. To reduce postage costs, we did not re‐send the hard copy questionnaire. Despite including the weblink for the questionnaire in the reminder letter, nearly two‐thirds of late responders responded in hard copy, indicating they had saved the questionnaire from the initial mail‐out for more than 2 weeks. The demographic and health characteristics of early and late responders were similar.
The cost of the mail‐out, $76 per participant, was similar to that in a pilot study which assessed the feasibility of recruiting participants with the same method for a research study in women's health ($101 per participant).26 Facebook advertising has been reported to be effective in smaller studies,16,17,27 and was relatively inexpensive in our large study, but it did not increase recruitment of people in the age group we targeted (55–60 years).
Limitations
The main barriers to increasing the number of study sites were financial in nature, but the number of experienced cardiovascular clinical trial sites interested in participating was also limited. We needed to balance the costs of each mail‐out against the time required to process the responses. We could not extend the recruitment period by another year, mainly for financial reasons. The decision at the end of 2016 to increase recruitment at the remaining four sites, rather than to recruit more sites, was again based on financial considerations, as each participating site received nearly $50 000 for training and start‐up costs.
Conclusions
On the basis of our experience with recruiting participants for AUSPICE, we recommend employing a mixture of recruitment methods when possible, providing both online and printed form options for responses, and sending reminder letters to boost response rates. The demographic and relevant clinical features of early and late responders eventually included in the study were similar. Social media advertising was not effective in increasing recruitment from our target age group of older adults.
Data sharing
The authors have full access to all de‐identified data (including statistical reports and tables) from the study. We do not have permission to supply de‐identified data for individual participants. Further data may be obtained by application to the study investigators. Additional study‐related information is available from the Australian New Zealand Clinical Trials Registry (ACTRN12615000536561).
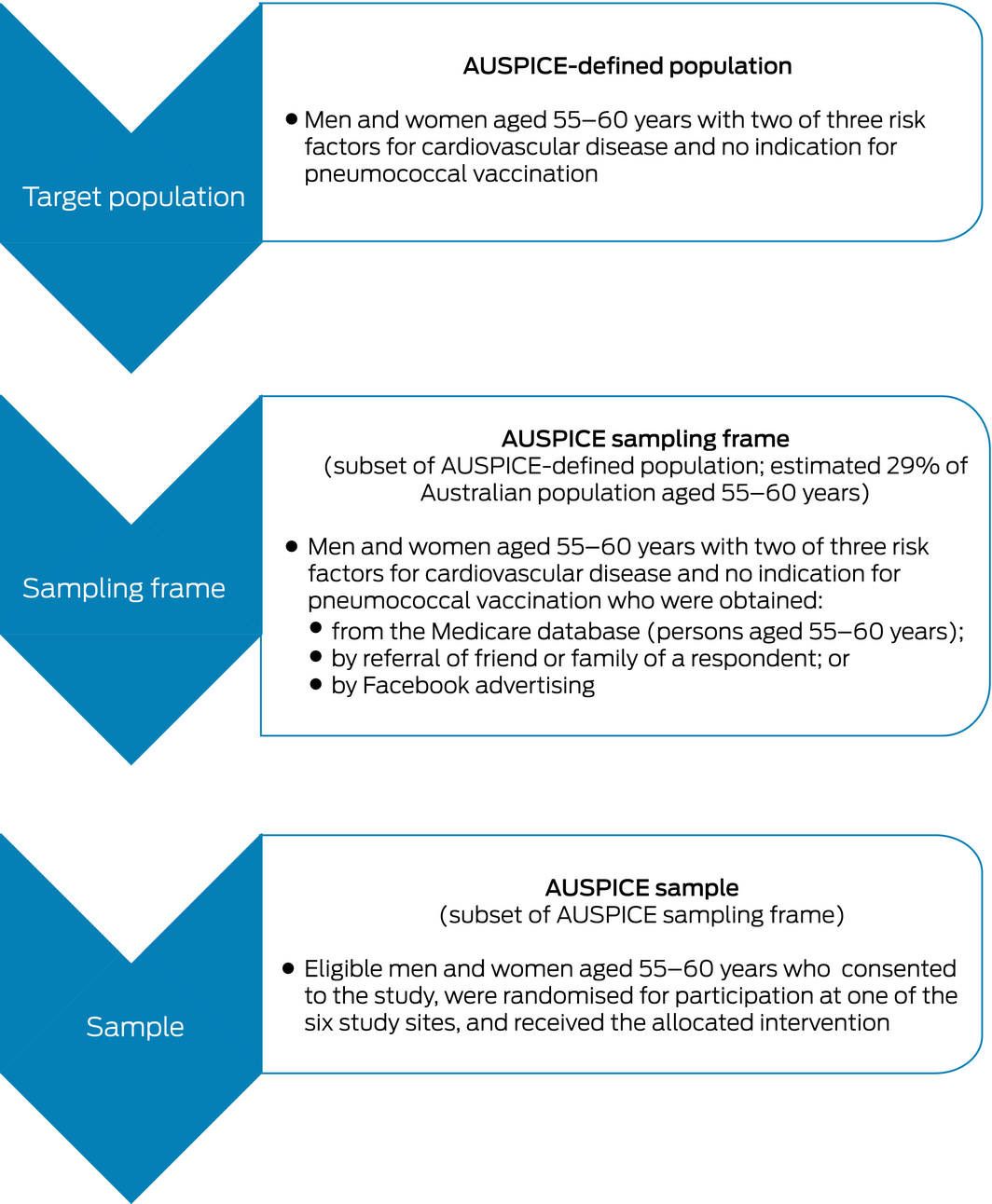
Box 1 – Sampling process for the Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE)
Box 2 – Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE): participation rates for all invitees and for invited persons eligible for study
|
Site |
Mail‐out population |
Expected eligible persons* |
Screening completed |
Eligible and randomised |
|||||||||||
|
Number of people |
Overall participation rate |
Eligible participation rate |
Number of people |
Overall participation rate |
Eligible participation rate |
||||||||||
|
|
|||||||||||||||
|
Adelaide |
27 500 |
8077 |
2157 |
7.8% |
27% |
683 |
2.5% |
8.5% |
|||||||
|
Canberra |
27 498 |
8077 |
2982 |
11% |
37% |
1244 |
4.5% |
15% |
|||||||
|
Gosford |
17 499 |
5140 |
1442 |
8.2% |
28% |
454 |
2.6% |
8.8% |
|||||||
|
Melbourne |
32 500 |
9546 |
2916 |
9.0% |
31% |
812 |
2.5% |
8.5% |
|||||||
|
Newcastle |
32 496 |
9545 |
4277 |
13% |
45% |
1175 |
3.6% |
12% |
|||||||
|
Perth |
17 499 |
5140 |
1465 |
8.4% |
29% |
357 |
2.0% |
6.9% |
|||||||
|
Unknown† |
— |
— |
6287 |
— |
— |
— |
— |
— |
|||||||
|
Total |
154 992 |
45522 |
21 526 |
14% |
47% |
4725 |
3.0% |
10% |
|||||||
|
|
|||||||||||||||
|
* Estimated: 29% of total mail‐out population, based on findings of the Hunter Community Study in Newcastle.3. † These respondents did not provide their postcode or contact details, could therefore not be followed up to organise a clinic visit. ◆ |
|||||||||||||||
Box 3 – Flow chart of the Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE)
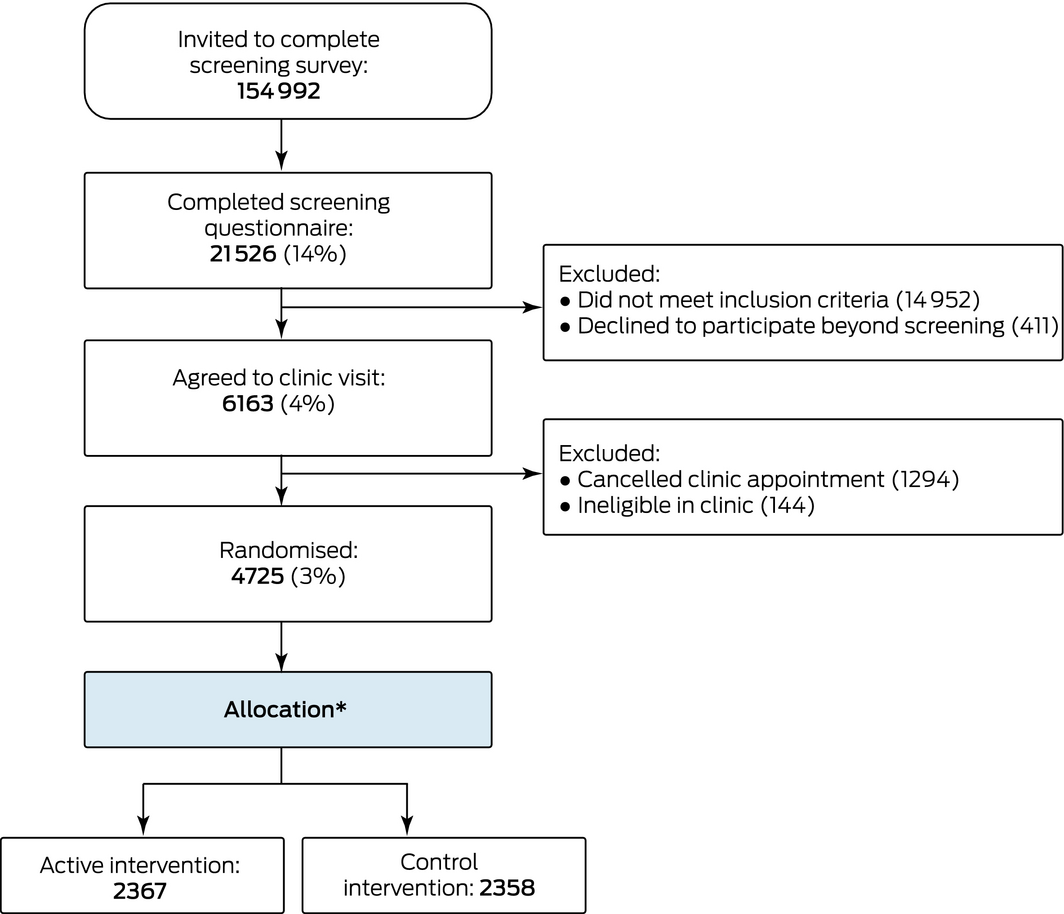
* All participants received the allocated intervention. ◆
Box 4 – Characteristics of participants in the Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE), by early and late response to the screening questionnaire
|
Characteristic |
All responders |
Early responders |
Late responders |
P |
|||||||||||
|
|
|||||||||||||||
|
Number of responders |
4697* |
746 [15.9%] |
3951 [84.1%] |
|
|||||||||||
|
City |
|
|
|
< 0.001 |
|||||||||||
|
Adelaide |
683 (15%) |
124 [18%] |
559 [82%] |
|
|||||||||||
|
Canberra |
1244 (26%) |
197 [16%] |
1047 [84%] |
|
|||||||||||
|
Gosford |
454 (9.7%) |
45 [10%] |
409 [90%] |
|
|||||||||||
|
Melbourne |
784 (17%) |
127 [16%] |
657 [84%] |
|
|||||||||||
|
Newcastle |
1175 (25%) |
213 [18%] |
962 [76%] |
|
|||||||||||
|
Perth |
357 (7.6%) |
40 [11%] |
317 [89%] |
|
|||||||||||
|
Sex |
|
|
|
0.65 |
|||||||||||
|
Women |
2275 (48%) |
367 [16%)] |
1908 [84%] |
|
|||||||||||
|
Men |
2422 (52%) |
379 [16%] |
2043 [84%] |
|
|||||||||||
|
Participant response |
|
|
|
< 0.001 |
|||||||||||
|
Online form |
1782 (38%) |
384 (51%) |
1398 (35%) |
|
|||||||||||
|
Paper form |
2915 (62%) |
362 (49%) |
2553 (65%) |
|
|||||||||||
|
High blood pressure |
|
|
|
0.20 |
|||||||||||
|
Yes |
3316 (71%) |
547 (73%) |
2769 (70%) |
|
|||||||||||
|
No |
1368 (29%) |
197 (26%) |
1171 (30%) |
|
|||||||||||
|
Unsure |
13 (0.3%) |
2 (0.3%) |
11 (0.3%) |
|
|||||||||||
|
High cholesterol level |
|
|
|
0.15 |
|||||||||||
|
Yes |
3314 (71%) |
507 (68%) |
2807 (71%) |
|
|||||||||||
|
No |
1348 (29%) |
235 (32%) |
1113 (28%) |
|
|||||||||||
|
Unsure |
35 (0.7%) |
4 (0.5%) |
31 (0.8%) |
|
|||||||||||
|
Overweight |
|
|
|
0.17 |
|||||||||||
|
Yes |
3007 (64%) |
480 (64%) |
2527 (64%) |
|
|||||||||||
|
No |
1128 (24%) |
178 (24%) |
950 (24%) |
|
|||||||||||
|
Unsure |
221 (4.7%) |
44 (5.9%) |
177 (4.5%) |
|
|||||||||||
|
Missing data† |
341 (7.3%) |
44 (5.9%) |
297 (7.5%) |
|
|||||||||||
|
Age (years), mean (SD) |
58.1 (1.7) |
58.0 (1.8) |
58.1 (1.7) |
0.11 |
|||||||||||
|
Blood pressure, diastolic (mmHg), mean (SD) |
85 (12) |
85 (10) |
85 (12) |
0.93 |
|||||||||||
|
Blood pressure, systolic (mmHg), mean (SD) |
138 (17) |
139 (16) |
138 (17) |
0.12 |
|||||||||||
|
Pulse (bpm), mean (SD) |
75 (12) |
77 (13) |
75 (12) |
< 0.001 |
|||||||||||
|
Height (cm), mean (SD) |
170 (10) |
170 (11) |
170 (10) |
0.69 |
|||||||||||
|
Waist circumference (cm), mean (SD) |
105 (14) |
106 (17) |
105 (13) |
0.46 |
|||||||||||
|
Weight (kg), mean (SD) |
92 (29) |
94 (55) |
92 (22) |
0.11 |
|||||||||||
|
|
|||||||||||||||
|
All percentages are column percentages, except for those in square brackets (row percentages). * 28 participants for whom baseline data were incomplete are not included in this table. † Responders met eligibility criteria (hypertension and high cholesterol), so overweight status was not captured. All participants were weighed during their clinic or randomisation visit. ◆ |
|||||||||||||||
Box 5 – Participation rates by Index of Relative Socio‐economic Disadvantage (IRSD) quintile
|
IRSD quintile* |
Number approached |
Number of participants |
Participation rate |
||||||||||||
|
|
|||||||||||||||
|
1 (most disadvantaged) |
6651 |
133 |
2.0% |
||||||||||||
|
2 |
25 725 |
781 |
3.0% |
||||||||||||
|
3 |
25 805 |
806 |
3.1% |
||||||||||||
|
4 |
34 615 |
1059 |
3.1% |
||||||||||||
|
5 (least disadvantaged) |
62 060 |
1893 |
3.1% |
||||||||||||
|
|
|||||||||||||||
|
* Missing data: postcodes were not recorded for 136 invitees, including 25 participants. χ2 test for independence (excluding participants without postcode data): P < 0.001. ◆ |
|||||||||||||||
Received 15 September 2018, accepted 24 January 2019
- Roseanne Peel1
- Shu Ren1
- Alexis Hure1
- Tiffany‐Jane Evans2
- Catherine A D'Este3
- Walter P Abhayaratna4
- Andrew M Tonkin5
- Ingrid Hopper5
- Amanda G Thrift5
- Christopher R Levi1
- Jonathan Sturm1
- David Durrheim1
- Joseph Hung6,7
- Tom G Briffa7
- Derek P Chew8
- Phil Anderson9
- Lynelle Moon9
- Mark McEvoy1
- Philip M Hansbro1,10
- David A Newby1
- John R Attia1,11
- 1 University of Newcastle, Newcastle, NSW
- 2 Hunter Medical Research Institute, Newcastle, NSW
- 3 National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT
- 4 Australian National University Medical School, Canberra Hospital, Canberra, ACT
- 5 Monash University, Melbourne, VIC
- 6 Sir Charles Gairdner Hospital, Perth, WA
- 7 University of Western Australia, Perth, WA
- 8 Flinders University, Adelaide, SA
- 9 Australian Institute of Health and Welfare, Canberra, ACT
- 10 Centenary UTS Centre for Inflammation, Sydney, NSW
- 11 Centre for Clinical Epidemiology and Biostatistics, University of Newcastle, Newcastle, NSW
The study was funded by a National Health and Medical Research Council (NHMRC) project grant (APP1062563). Ingrid Hopper and Philip Hansbro were supported by NHMRC fellowships.
No relevant disclosures.
- 1. O'Toole J, Rodrigo S, Sinclair M, Leder K. The Australian Electoral Commission Roll has good utility for “niche” household recruitment in population health studies. Aust N Z J Public Health 2009; 33: 137–139.
- 2. Smith W, Mitchell P, Attebo K, Leeder S. Selection bias from sampling frames: telephone directory and electoral roll compared with door‐to‐door population census: results from the Blue Mountains Eye Study. Aust N Z J Public Health 1997; 21: 127–133.
- 3. McEvoy M, Smith W, D'Este C, et al. Cohort profile: the Hunter Community Study. Int J Epidemiol 2010; 39: 1452–1463.
- 4. Brown WJ, Bryson L, Byles JE, et al. Women's Health Australia: recruitment for a national longitudinal cohort study. Women Health 1998; 28: 23–40.
- 5. Loff B, Campbell EA, Glass DC, et al. Access to the Commonwealth electoral roll for medical research. Med J Aust 2013; 199: 128–130. https://www.mja.com.au/journal/2013/199/2/access-commonwealth-electoral-roll-medical-research
- 6. The 45 and Up Study Collaborators. Cohort profile: the 45 and Up Study. Int J Epidemiol 2008; 37: 941–947.
- 7. Mindell JS, Giampaoli S, Goesswald A, et al. Sample selection, recruitment and participation rates in health examination surveys in Europe: experience from seven national surveys. BMC Med Res Methodol 2015; 15: 78.
- 8. Sully BGO, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013; 14: 166.
- 9. Derrick JL, Eliseo‐Arras RK, Hanny C, et al. Comparison of internet and mailing methods to recruit couples into research on unaided smoking cessation. Addict Behav 2017; 75: 12–16.
- 10. Morgan AJ, Jorm AF, Mackinnon AJ. Internet‐based recruitment to a depression prevention intervention: lessons from the Mood Memos study. J Med Internet Res 2013; 15: e31.
- 11. Bower P, Brueton V, Gamble C, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014; 15: 399.
- 12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta‐analysis. BMJ Open 2013; 3: e002360.
- 13. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009; MR000008.
- 14. Treweek S, Altman DG, Bower P, et al. Making randomised trials more efficient: report of the first meeting to discuss the Trial Forge platform. Trials 2015; 16: 261.
- 15. Ding EL, Powe NR, Manson JE, et al. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med 2007; 167: 905–912.
- 16. Mishra GD, Hockey R, Powers J, et al. Recruitment via the Internet and social networking sites: the 1989–1995 cohort of the Australian Longitudinal Study on Women's Health. J Med Internet Res 2014; 16: e279.
- 17. Alley S, Jennings C, Plotnikoff RC, Vandelanotte C. An evaluation of web‐ and print‐based methods to attract people to a physical activity intervention. JMIR Res Protoc 2016; 5: e94.
- 18. Cowie JM, Gurney ME. The use of Facebook advertising to recruit healthy elderly people for a clinical trial: baseline metrics. JMIR Res Protoc 2018; 7: e20.
- 19. Ren S, Hure A, Peel R, et al. Rationale and design of a randomized controlled trial of pneumococcal polysaccharide vaccine for prevention of cardiovascular events: the Australian Study for the Prevention through Immunization of Cardiovascular Events (AUSPICE). Am Heart J 2016; 177: 58–65.
- 20. Australian Bureau of Statistics. 2033.0.55.001. Census of population and housing: Socio‐Economic Indexes for Areas (SEIFA), Australia, 2016. Postal area, indexes, SEIFA 2016; table 2. Updated 27 Mar 2018. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012016?OpenDocument (viewed Feb 2019).
- 21. Akmatov MK, Jentsch L, Riese P, et al. Motivations for (non)participation in population‐based health studies among the elderly: comparison of participants and nonparticipants of a prospective study on influenza vaccination. BMC Med Res Methodol 2017; 17: 18.
- 22. Hartge P. Participation in population studies. Epidemiology 2006; 17: 252–254.
- 23. Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007; 17: 643–653.
- 24. Detoc M, Gagneux‐Brunon A, Lucht F, Botelho‐Nevers E. Barriers and motivations to volunteers’ participation in preventive vaccine trials: a systematic review. Expert Rev Vaccines 2017; 16: 467–477.
- 25. Cook DA, Wittich CM, Daniels WL, et al. Incentive and reminder strategies to improve response rate for internet‐based physician surveys: a randomized experiment. J Med Internet Res 2016; 18: e244.
- 26. Harris ML, Herbert D, Loxton D, et al. Recruiting young women for health surveys: traditional random sampling methods are not cost‐effective. Aust N Z J Public Health 2014; 38: 495.
- 27. Gilligan C, Kypri K, Bourke J. Social networking versus Facebook advertising to recruit survey respondents: a quasi‐experimental study. JMIR Res Protoc 2014; 3: e48.





Abstract
Objectives: To examine the effectiveness of different strategies for recruiting participants for a large Australian randomised controlled trial (RCT), the Australian Study for the Prevention through Immunisation of Cardiovascular Events (AUSPICE).
Design, setting, participants: Men and women aged 55–60 years with at least two cardiovascular risk factors (hypertension, hypercholesterolaemia, overweight/obesity) were recruited for a multicentre placebo‐controlled RCT assessing the effectiveness of 23‐valent pneumococcal polysaccharide vaccine (23vPPV) for preventing cardiovascular events.
Methods: Invitations were mailed by the Australian Department of Human Services to people in the Medicare database aged 55–60 years; reminders were sent 2 weeks later. Invitees could respond in hard copy or electronically. Direct recruitment was supplemented by asking invitees to extend the invitation to friends and family (snowball sampling) and by Facebook advertising.
Main outcome: Proportions of invitees completing screening questionnaire and recruited for participation in the RCT.
Results: 21 526 of 154 992 invited people (14%) responded by completing the screening questionnaire, of whom 4725 people were eligible and recruited for the study. Despite the minimal study burden (one questionnaire, one clinic visit), the overall participation rate was 3%, or an estimated 10% of eligible persons. Only 16% of eventual participants had responded within 2 weeks of the initial invitation letter (early responders); early and late responders did not differ in their demographic or medical characteristics. Socio‐economic disadvantage did not markedly influence response rates. Facebook advertising and snowball sampling did not increase recruitment.
Conclusions: Trial participation rates are low, and multiple concurrent methods are needed to maximise recruitment. Social media strategies may not be successful in older age groups.
Trial registration: Australian New Zealand Clinical Trials Registry, ACTRN12615000536561.