Australia has witnessed dramatic improvement in asthma outcomes over the past 20 years. Asthma death rates have improved considerably, and fewer patients are presenting to hospital emergency departments or being admitted to hospital.1 Similar improvements in asthma have occurred in other well resourced countries around the world. Improved patient education, a focus on preventing asthma attacks, written action plans, regular use of inhaled corticosteroids (ICS) to suppress airway inflammation, and availability of long-acting bronchodilators have all contributed to improved outcomes.
However, there remains a group of patients who continue to experience poor asthma control, multiple asthma exacerbations and frequent oral steroid use, despite what appears to be optimal therapy with high dose ICS and long-acting β2-agonists (LABA), corresponding to Step 4 of the Global Initiative for Asthma (GINA) guidelines2 or the upper treatment tier in the Australian Asthma Handbook (www.asthmahandbook.org.au). This article summarises the key evidence around new and emerging medications for severe asthma; ie, patients who are at Step 5 of the GINA guidelines2 (Box 1).
PubMed searches were performed using the search terms “severe asthma” or “difficult asthma”, “treatment”, “monoclonal antibodies” and “randomised controlled trials”. Guidelines, position statements, reviews and original articles were reviewed.
Principles of clinical assessment
Before progressing to complex or expensive add-on therapies, it is important that the treating doctor ask a number of questions, as highlighted in Box 2 and as discussed in further detail elsewhere in this supplement.3,4 Consider referral to a specialist with experience in severe asthma if expert advice is needed to address these issues. Primary care has an important and ongoing role in severe asthma, as discussed in more detail by Chung and colleagues in this supplement.5
Heterogeneity in asthma and addressing treatable traits
Over the past 10 to 20 years, there has been tremendous progress in understanding asthma pathophysiology. It has become recognised that asthma is not a single disease, but rather a clinical syndrome that reflects diverse patterns of airway inflammation.6,7 Researchers are defining several different asthma phenotypes, based on the age of onset, the presence of allergic sensitisation, type of inflammation (eosinophilic or neutrophilic), obesity, smoking and other environmental exposures.8 These phenotypes are likely to reflect distinct molecular mechanisms,9,10 and in the future are likely to form the basis for personalised asthma medicines. Targeted therapies are likely to be expensive in the short term, so the one-size-fits-all treatment approach of ICS with or without LABA will likely remain the norm in mild to moderate asthma, with targeted therapies reserved for patients with severe asthma that is refractory to conventional treatments. Further, a major focus among severe asthma specialists and severe asthma clinics is the identification of treatable traits; ie, aspects of an individual patient that can be identified and addressed in order to improve clinical outcomes.11 Treatable traits include processes involving the lower airway, the upper airway and extra-pulmonary disorders that indirectly impact on asthma outcomes, and include such features as airway eosinophilia, persistent airflow obstruction, a history of frequent asthma flare-ups, persistent airway infection or bacterial colonisation, sinonasal disease, obstructive sleep apnoea and obesity.11 Identifying such problems can guide the choice of appropriate add-on therapies (Box 3). For example, blood or airway eosinophilia is usually a good indicator of corticosteroid-responsive disease.8,12
Add-on therapies
Long-acting anti-muscarinic antagonists
Acetylcholine causes smooth muscle constriction, and long-acting anti-muscarinic antagonists (LAMA) have long been used in chronic obstructive pulmonary disease but not in asthma. Large randomised controlled trials have now shown that adding tiotropium to ICS/LABA combination therapy in exacerbation-prone asthma patients with persistent airflow obstruction improves lung function and delays asthma exacerbations.13,14 However, the benefits of tiotropium seem incremental rather than transformative, with modest improvements in symptoms and quality of life. Tiotropium administered by mist inhaler is now available in Australia under the Pharmaceutical Benefits Scheme (PBS) as add-on therapy in asthma where there has been at least one exacerbation in the past 12 months despite optimised asthma therapy (ICS/LABA, including assessment of correct inhaler technique and optimising adherence). Other LAMA may work in severe asthma, but robust evidence is lacking, and the PBS does not currently support the use of other LAMA in asthma.
Leukotriene receptor antagonists
Leukotrienes are a family of lipid mediators that contribute to airway inflammation and smooth muscle contraction. Montelukast is a leukotriene receptor antagonist, and some evidence supports its use as an add-on to ICS in moderate asthma,15 although it is not clear whether a higher dose of ICS would achieve a similar clinical improvement.15 Aspirin-intolerant asthma is thought to be mediated by leukotrienes, and randomised controlled trials indicate that montelukast is likely to be beneficial where there is clear evidence of aspirin sensitivity.16 Recurrent nasal polyps may occur in conjunction with asthma and aspirin sensitivity (Samter’s triad). However, the available data do not support the use of montelukast in unselected patients with severe asthma,17 and there is no evidence to support long term use of montelukast if there is no clear symptomatic improvement after a 1-month treatment trial.18
Continuous oral corticosteroids
Once traditional therapies have been exhausted, clinical guidelines frequently recommend progression to long term oral corticosteroids (eg, Step 5 in the GINA guidelines2). Blood eosinophilia is a reasonable indicator of steroid-responsive disease, and a recent study has shown that the blood eosinophil count can be used to titrate the oral steroid dose in this situation.12 Wark and colleagues used a treatment algorithm that kept the blood eosinophil count below 0.2 × 109/L, and this led to a reduction in asthma exacerbations and better symptom control.12 Although continuous oral steroids are often very effective at re-establishing asthma control, their use comes at the cost of substantial side effects, including weight gain, osteoporosis, mood change, mental health problems, sleep disturbance, cataracts and glucose intolerance.19 The risk–benefit equation should be discussed openly with the patient and family. Identification of patients with steroid-dependent asthma should prompt specialist referral for reassessment and consideration of other treatments, even if current symptoms appear minimal. The recent emergence of effective targeted therapies which appear to have limited side effects raises questions about the place of long term oral steroids in asthma management in the modern era, given their significant adverse effects. This is discussed further elsewhere in this supplement.20
Antifungal agents
Patients with severe asthma are more likely to be sensitised to airborne fungi than those with mild or moderate asthma, and the presence of serum IgE antibodies against specific fungi should be assessed by in vitro testing or skin prick tests. A proportion will have particular features that suggest allergic bronchopulmonary aspergillosis, such as mucous plugging, central bronchiectasis on computed tomography of the chest, very high total and specific IgE, and blood eosinophilia. There are some clinical trial data to support the use of itraconazole in severe asthma with fungal sensitisation21 and in allergic bronchopulmonary aspergillosis.22 However, the risk of side effects and clinically important adrenal suppression due to interactions with ICS and other medications must be taken into account. Itraconazole and related antifungal agents are not approved by the Therapeutic Goods Administration (TGA) or funded by the PBS for treatment of asthma, although severe asthma clinics will often initiate a 4-month therapeutic trial of off-label itraconazole in selected patients.18
Theophylline
Theophylline can be used as an add-on therapy, and older studies in moderate asthma showed that adding theophylline to low dose ICS improved lung function and rescue bronchodilator use.23 However, LABA may be more effective bronchodilators, and very few studies have examined the use of theophylline in severe asthma.24 Side effects and drug interactions are important considerations. Current international guidelines do not recommend theophylline in severe asthma.25
Immunosuppressive therapy
Although immunosuppressive interventions have been proposed as a corticosteroid-sparing strategy, the evidence base is weak. Methotrexate may have a small oral steroid-sparing effect, with a mean reduction in prednisone/prednisolone doses across multiple trials of 3–4 mg per day.26 However, current American and European guidelines recommend against the use of methotrexate in adults or children with severe asthma.25 This was a conditional recommendation, based on low quality evidence, which highlighted the limited benefits of treatment and the potential for adverse events.25 Owing to negative side effects, the risk–benefit balance requires careful consideration. Mycophenolate mofetil may be effective in severe asthma, although the only evidence comes from observational studies,27 and there is a need for randomised controlled trials (RCTs) to properly evaluate the place of this agent in severe asthma management. Methotrexate and mycophenolate mofetil are not TGA approved or PBS funded for treatment of asthma.
Macrolide antibiotics
Macrolide antibiotics (eg, azithromycin) have immunomodulatory, anti-inflammatory and anti-microbial effects that may be of benefit in severe asthma. A recent RCT of individuals with persistent uncontrolled asthma despite ICS in combination with long-acting bronchodilators showed that azithromycin for 48 weeks reduced exacerbations and improved quality of life.28 People with prolonged QT interval on electrocardiogram and those with hearing impairment were excluded. The potential for antibiotic resistance with widespread use remains a concern, and severe asthma is not currently a TGA approved or PBS funded indication for long term azithromycin. Ongoing research is examining which severe asthma patients are most likely to benefit from macrolides and the optimal duration of treatment.
Allergen immunotherapy
A recent large RCT found that sublingual immunotherapy reduced asthma exacerbations in house dust mite allergic asthma29 but had little impact on symptom control. The study predominantly recruited patients with moderate rather than severe asthma. GINA guidelines recommend that clinicians consider sublingual immunotherapy as add-on therapy in adult house dust mite-sensitive patients with allergic rhinitis who have exacerbations despite ICS treatment, provided the forced expiratory volume in 1 second is > 70% predicted.2 In our view, further research is needed before the place of sublingual immunotherapy in severe asthma is fully established, especially in patients with poor lung function.29
Biologicals and other novel targeted therapies
A number of monoclonal antibodies are now available in Australia for severe asthma treatment. These biologicals target molecular pathways that contribute to asthma pathogenesis and can be transformative in selected patients, markedly reducing the frequency of asthma exacerbations and allowing many to reduce or eliminate their use of long term oral corticosteroids. More such agents are likely to emerge in the near future. These biologicals are expensive, so it is important that they are targeted to those most likely to respond. Prescription of biologicals can only be initiated by specialists and requires a detailed written or online submission to the PBS. Further details can be found on the websites of the PBS (www.pbs.gov.au) and the Centre of Excellence in Severe Asthma (www.severeasthma.org.au).
The PBS currently requires that monoclonal antibodies can only be prescribed for severe asthma patients who have been under specialist care for 6–12 months. Specialists will often use this period to try one or more of the preceding add-on therapies before proceeding to monoclonal antibody treatment. There is no evidence base to guide the most appropriate treatment sequence.
Biologicals targeting IgE
The IgE pathway is central to the pathogenesis of allergic diseases, including allergic asthma. Omalizumab is a recombinant humanised monoclonal antibody targeting the fragment crystallisable region of IgE. It acts rapidly to block cross-linking of IgE receptors on mast cells and basophils, preventing allergic activation, and gradually down regulates IgE receptor expression on various immune cells.
The effectiveness and safety of omalizumab in treatment-refractory severe allergic asthma is well established.30 Omalizumab treatment reduces exacerbation rates by about 45% and hospitalisations by over 80%.31 It improves asthma control and quality of life when added to ICS/LABA therapy. Improvements in lung function tend to be small and have not been consistently demonstrated in the trials.31 Omalizumab may also permit reductions in ICS doses or even cessation of ICS entirely,32 although whether the latter is a desirable outcome remains contentious.
The drug is mostly well tolerated, with local skin reactions the most common adverse effect. Features that are characteristic of many monoclonal antibodies, such as headaches, lethargy and anti-drug antibodies, seem uncommon. Anaphylaxis is reported in about 0.2% of patients, and is most likely after the initial doses of omalizumab, particularly in the first 30–60 minutes after a dose, or in those with previous anaphylaxis.33 Patients and health care workers should be aware of the clinical features and management of anaphylaxis.
Omalizumab is available in Australia via the PBS as an add-on treatment for severe allergic asthma. Eligibility criteria are outlined in Box 4. The drug is given by subcutaneous injections every 2 or 4 weeks, with the dose calculated based on the total IgE concentration and body weight.
Only respiratory physicians, clinical immunologists and physicians with a special interest in asthma can prescribe omalizumab, and this requires a detailed written or online submission to the PBS. Approval is given for an initial trial of therapy, with ongoing supply dependent on good evidence of a treatment response. Clinical improvement usually takes a few months to become evident. If no improvement is seen by 6 months, it is very unlikely that the individual will respond. The future is likely to see additional monoclonal antibodies that target IgE.
Biologicals targeting eosinophils
Interleukin-5 (IL-5) is the key cytokine that promotes eosinophil differentiation, activation and survival, and there are a number of compounds available now or in late stage clinical development for severe eosinophilic asthma. Mepolizumab and reslizumab are monoclonal antibodies that target IL-5, whereas benralizumab targets the IL-5 receptor.
The early clinical trials that targeted IL-5 highlighted the importance of selecting the appropriate asthma phenotype. Initial studies with mepolizumab and reslizumab in unselected asthma patients proved disappointing.34,35 It was only when mepolizumab and reslizumab were used in exacerbation prone patients with severe eosinophilic asthma that their effectiveness was fully realised.36-38 Biologicals targeting eosinophils may be especially effective in patients with severe asthma plus recurrent nasal polyps.39,40
Mepolizumab
Mepolizumab reduces eosinophil numbers in blood and sputum, reduces the frequency of asthma exacerbations37 and hospital admissions,41 and improves asthma symptoms and quality of life.42 It has an oral steroid-sparing role43 and may induce a modest improvement in lung function.44
Initially, mepolizumab was targeted to asthma patients with high numbers of sputum eosinophils, but it is now clear that the blood eosinophil count provides a reasonable and practical predictor of sputum eosinophils.45 Response to mepolizumab is most likely when blood eosinophils are ≥ 0.3 × 109/L, a level that is within the quoted normal range in most laboratories.46 Further, the effect size varies in relation to the blood eosinophil count, with the greatest reductions in asthma exacerbations seen in patients with the highest blood eosinophil counts.37,46
The drug is mostly well tolerated, with local skin reactions the most common adverse effect. Headaches, lethargy and anti-drug antibodies are reported, but seem uncommon. In patients with very high eosinophil counts, clinicians should consider the possibility of the multi-system disorder eosinophilic granulomatosis with polyangiitis (formerly known as Churg–Strauss syndrome) or occult helminth infection.
Mepolizumab is available in Australia via the PBS as an add-on treatment for the management of severe eosinophilic asthma. Eligibility criteria are outlined in Box 4. The drug is given in a dose of 100 mg by subcutaneous injections every 4 weeks.
Benralizumab
Benralizumab interferes with the binding of IL-5 to its receptor and induces antibody-mediated cell cytotoxicity of eosinophils and basophils. This leads to almost complete depletion of blood eosinophil counts, much greater than is seen with mepolizumab and reslizumab. Whether this translates to greater clinical efficacy remains to be determined. There is good evidence from phase 3 studies that benralizumab reduces asthma exacerbations, improves asthma symptoms, quality of life and spirometry,47,48 and has a strong corticosteroid-sparing effect.49 The initial three injections of benralizumab are given subcutaneously every 4 weeks, but thereafter the dosing interval can be extended to 8 weeks. It is approved by the TGA, has recently been recommended for funding by the Pharmaceutical Benefits Advisory Committee, and is currently available in Australia via a patient access program.
Reslizumab
Large RCTs have demonstrated that intravenous reslizumab reduces asthma exacerbations and improves symptom scores, quality of life and spirometry.38,39 Intravenous reslizumab is currently approved by the TGA but is not currently available via the PBS. Its manufacturer recently announced that two phase 3 studies of subcutaneous reslizumab in severe eosinophilic asthma failed to meet their primary endpoint of reducing asthma exacerbation frequency.50 The place of reslizumab in asthma treatment is therefore unclear at present.
Other targeted therapies under development
Dupilumab is a monoclonal antibody that blocks the common receptor for IL-4 and IL-13, two cytokines involved in asthma and allergic inflammation. In a phase 2b RCT, dupilumab added to ICS/LABA improved lung function and reduced severe asthma exacerbations, irrespective of the baseline eosinophil count.51 Dupilumab may also be effective in patients with nasal polyps40 or atopic dermatitis.52 Phase 3 studies of dupilumab in asthma have not yet been reported at the time of publication, and dupilumab is not currently licensed for use in asthma.
Tezepelumab is a monoclonal antibody directed against thymic stromal lymphopoietin, a cytokine produced by epithelial cells with effects on multiple immune cells. Thymic stromal lymphopoietin is highly expressed in the airways in asthma, and a recent phase 2 RCT showed that addition of tezepelumab to ICS/LABA reduced asthma exacerbation rates by 60–70% over placebo, regardless of baseline blood eosinophil counts.53 Lung function also improved in tezepelumab-treated participants. Tezepelumab is not currently licensed for use in asthma.
Fevipiprant is an orally available agent that blocks the prostaglandin D2 receptor CRTH2. One RCT has shown that it reduces airway eosinophilia in persistent moderate-to-severe asthma and sputum eosinophilia despite ICS treatment.54 Further evidence is required before its place in asthma management is established, but because it is a small molecule given by mouth rather than injection, it may be cheaper to manufacture than monoclonal antibodies.
Bronchial thermoplasty is a procedure that involves the delivery of controlled radiofrequency energy to the airway wall during bronchoscopy, thus heating the tissue and reducing the amount of airway smooth muscle. A large sham controlled RCT showed fewer asthma exacerbations and improved quality of life, although there were large improvements in the sham arm of the study.55 Despite targeting airway smooth muscle, thermoplasty surprisingly has little if any impact on lung function.56 The procedure involves three sequential bronchoscopies, and although there is often a short term deterioration in asthma control, no long term safety issues have emerged thus far.56 It is not clear how best to identify suitable candidates for thermoplasty, and an expert European and American task force25 has recommended that patients who undergo thermoplasty should be enrolled in a clinical trial or registry to carefully document real world outcomes after this procedure. Thermoplasty is not yet recommended in the GINA guidelines or the Australian Asthma Handbook.
Conclusions and questions for the future
A number of new, exciting treatment options for severe asthma have become available in recent years, and this is likely to expand in the near future, with treatments that have a major impact on asthma exacerbations, quality of life and the need for long term oral corticosteroids. Many new medications are being developed for allergic and eosinophilic variants of asthma, although fewer options are available for other inflammatory variants or where chronic structural changes in the airways produce fixed airway obstruction. Importantly, the ultimate goal of inducing remission or cure of asthma is probably some distance away. The basic principles of severe asthma management are summarised in Box 5.
Because the new monoclonal antibodies are expensive, both health care professionals and governments have a responsibility to ensure that they are used appropriately, and only after trying cheaper evidence-based treatment options. In some therapeutic areas such as pulmonary fibrosis, there is already a precedent in Australia that expensive treatments can only be prescribed on the consensus recommendation of an expert multidisciplinary team meeting, and in our view such an approach should be introduced in severe asthma.
Box 1 – Stepwise treatment of asthma, showing possible add-on therapies for particular asthma phenotypes
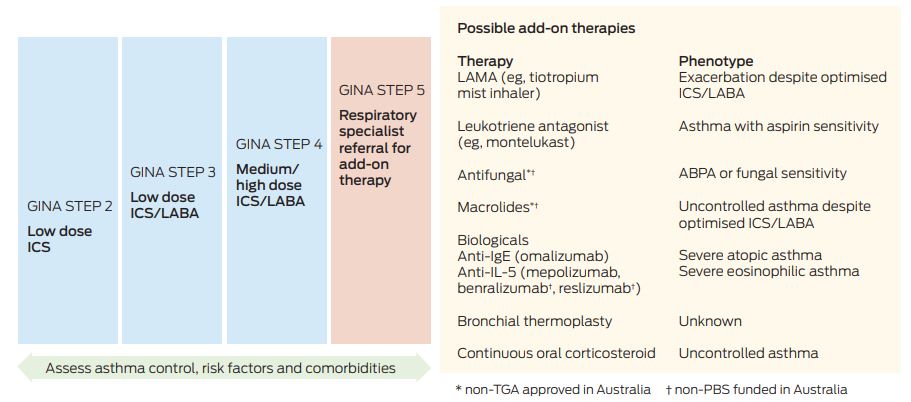
ABPA = allergic bronchopulmonary aspergillosis. GINA = Global Initiative for Asthma.2 IL-5 = interleukin-5. ICS = inhaled corticosteroids. LABA = long-acting β2-agonists. LAMA = long-acting anti-muscarinic antagonists. PBS = Pharmaceutical Benefits Scheme. TGA = Therapeutic Goods Administration.
Box 2 – Diagnostic questions to consider
- Are the clinical features consistent with asthma, or might the diagnosis be incorrect? Are additional investigations required?
- Is there evidence of variable airflow obstruction such as bronchodilator reversibility or peak flow variability of 15% or more? Is bronchial challenge testing or observation of the response to therapy over time required to confirm an asthma diagnosis?
- Even if the patient has asthma, can the current symptoms be solely attributed to asthma, or might they be due to other problems? Obesity, deconditioning, dysfunctional breathing, panic attacks and vocal cord dysfunction can mimic asthma, as discussed elsewhere in this supplement.3,4
- Has the inhaler technique been checked, is the preventer inhaler being used as prescribed, and have self-management skills been optimised? Poor inhaler technique and erratic adherence are common in patients referred to severe asthma clinics.
- Is an unrecognised environmental factor (eg, tobacco or marijuana smoking, or exposure to an agent in the workplace or a hobby) contributing to poor control?
- Have relevant comorbidities been managed?
Box 3 – Using treatable traits and asthma phenotypes to guide add-on therapies in severe asthma*
|
Treatable trait |
Investigation and treatment options |
||||||||||||||
|
|
|||||||||||||||
|
Airflow obstruction despite ICS/LABA |
LAMA |
||||||||||||||
|
Rhinosinusitis |
Nasal steroid spray, nasal lavage, ENT specialist review |
||||||||||||||
|
Gastro-oesophageal reflux |
Acid suppression, lifestyle modification |
||||||||||||||
|
Obstructive sleep apnoea |
Sleep study, weight loss, sleep physician review |
||||||||||||||
|
Obesity |
Weight loss |
||||||||||||||
|
Anxiety, depression |
Cognitive behaviour therapy, anxiolytics or anti-depressants, psychologist/psychiatrist review |
||||||||||||||
|
Persistent airway infection, colonisation |
Chest computed tomography scan to assess for bronchiectasis, short course antibiotics, prophylactic low dose macrolides |
||||||||||||||
|
Vocal cord dysfunction |
ENT and speech pathology review |
||||||||||||||
|
Dysfunctional breathing/hyperventilation |
Physiotherapy review, breathing retraining, |
||||||||||||||
|
Aspirin-sensitive asthma |
Montelukast, immunology review |
||||||||||||||
|
Severe allergic asthma |
Monoclonal antibody targeting IgE |
||||||||||||||
|
Severe asthma with high blood eosinophils |
Monoclonal antibody targeting interleukin 5 or its receptor |
||||||||||||||
|
Severe asthma with nasal polyps |
ENT review, montelukast, monoclonal antibody targeting IL-5 pathway or IgE |
||||||||||||||
|
|
|||||||||||||||
|
ICS = inhaled corticosteroids. ENT = ear, nose and throat. IL-5 = interleukin-5. LABA = long-acting β2-agonists. LAMA = long-acting anti-muscarinic antagonists.* Before moving to add-on therapies, it is important for the treating clinician to first review the diagnosis of asthma, and check inhaler technique, adherence and self-management skills. |
|||||||||||||||
Box 4 – Current Pharmaceutical Benefits Scheme prescribing criteria for monoclonal antibodies for severe asthma
|
Criterion |
Omalizumab |
Mepolizumab |
|||||||||||||
|
|
|||||||||||||||
|
Severe atopic asthma |
Yes |
No |
|||||||||||||
|
Severe eosinophilic asthma |
No |
Yes |
|||||||||||||
|
Confirmed asthma diagnosis |
Yes |
Yes |
|||||||||||||
|
High dose ICS/LABA |
Yes |
Yes |
|||||||||||||
|
High use of oral corticosteroids in the past 12 months |
Yes |
Yes |
|||||||||||||
|
Poor asthma control and high symptom burden |
Yes |
Yes |
|||||||||||||
|
Asthma exacerbations in the past 12 months |
Yes |
Yes |
|||||||||||||
|
|
|||||||||||||||
|
ICS = inhaled corticosteroids. LABA = long-acting β2-agonists. |
|||||||||||||||
Box 5 – Principles of severe asthma management
- Correct inhaler technique and adherence to regular inhaled corticosteroids with or without long-acting bronchodilators are the cornerstones of asthma treatment.
- All patients should be assessed for psycho-social, behavioural and medical problems that may be contributing to poor asthma control and asthma flare-ups, prior to consideration of add-on therapies.
- Phenotyping is helpful in severe asthma to select the right add-on therapy for the right person.
- Add-on therapies can be trialled in individual patients by comparing symptom scores before and after treatment for a defined period, at a time when nothing else is being changed (modified n-of-1 trials). There is little point in persisting with add-on or off-label therapies if there is no clear evidence of benefit.
- Biologicals (monoclonal antibodies) target specific molecular pathways and have a major impact on asthma exacerbations, asthma symptoms and quality of life in selected severe asthma patients, and may enable reduction or elimination of maintenance oral steroid use. Biologicals are now available in Australia for the treatment of severe allergic asthma and severe eosinophilic asthma, with additional agents likely to become available in the near future.
- Long term oral corticosteroid use should be minimised or avoided if possible.
Provenance: Commissioned; externally peer reviewed.
- John W Upham1,2
- Li Ping Chung3
- 1 Diamantina Institute, University of Queensland, Brisbane, QLD
- 2 Princess Alexandra Hospital, Brisbane, QLD
- 3 Fiona Stanley Hospital, Perth, WA
John Upham receives funding support from the National Health and Medical Research Council (NHMRC), and is a chief investigator on the NHMRC-funded Centre of Excellence in Severe Asthma.
John Upham has received advisory board fees and lecture fees from Novartis, GlaxoSmithKline, AstraZeneca, Menarini, Boehringer Ingelheim and Mundipharma. Li Ping Chung has received advisory board fees and lecture fees from GlaxoSmithKline, Astra Zeneca, Menarini and Boehringer Ingelheim.
- 1. Australian Institute of Health and Welfare. Asthma. https://www.aihw.gov.au/reports/asthma-other-chronic-respiratory-conditions/asthma/data (viewed May 2018).
- 2. Global Initiative for Asthma. Global strategy for asthma management and prevention (2018 update). http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/ (viewed May 2018).
- 3. Tay TR, Lee J W-Y, Hew M. Diagnosis of severe asthma. Med J Aust 2018; 209 (2 Suppl): S3-S10.
- 4. Bardin PG, Rangaswamy J, Yo SW. Managing comorbid conditions in severe asthma. Med J Aust 2018; 209 (2 Suppl): S11-S17.
- 5. Chung LP, Johnson P, Summers Q. Models of care for severe asthma: the role of primary care. Med J Aust 2018; 209 (2 Suppl): S34-S40.
- 6. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology 2006; 11: 54-61.
- 7. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008; 178: 218-224.
- 8. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716-725.
- 9. Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008; 372(9643): 1107-1119.
- 10. Wenzel SE. Emergence of biomolecular pathways to define novel asthma phenotypes. Type-2 immunity and beyond. Am J Respir Crit Care Med 2016; 55: 1-4.
- 11. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410-419.
- 12. Wark PA, McDonald VM, Gibson PG. Adjusting prednisone using blood eosinophils reduces exacerbations and improves asthma control in difficult patients with asthma. Respirology 2015; 20: 1282-1284.
- 13. Kerstjens HA, Casale TB, Bleecker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med 2015; 3: 367-376.
- 14. Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012; 367: 1198-1207.
- 15. Chauhan BF, Jeyaraman MM, Singh Mann A, et al. Addition of anti-leukotriene agents to inhaled corticosteroids for adults and adolescents with persistent asthma. Cochrane Database Syst Rev 2017; (3): CD010347.
- 16. Dahlen SE, Malmstrom K, Nizankowska E, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 9-14.
- 17. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet 2001; 357: 2007-2011.
- 18. McDonald VM, Vertigan AE, Gibson PG. How to set up a severe asthma service. Respirology 2011; 16: 900-911.
- 19. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016; 71: 339-346.
- 20. Ramsahai JM, Wark P. Appropriate use of oral corticosteroids in severe asthma. Med J Aust 2018; 209 (2 Suppl): S18-S21.
- 21. Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med 2009; 179: 11-18.
- 22. Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol 2003; 111: 952-957.
- 23. Evans DJ, Taylor DA, Zetterstrom O, et al. A comparison of low-dose inhaled budesonide plus theophylline and high-dose inhaled budesonide for moderate asthma. N Engl J Med 1997; 337: 1412-1418.
- 24. Wang Y, Chen P, Dai A, et al. Intervention studies of inhaled corticosteroids combined with long-acting theophylline or long-acting beta2-agonists in patients with moderate to severe asthma: a randomized, controlled study. Clin Ther 2016; 38: 2622-2627.e1.
- 25. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343-373.
- 26. Davies H, Olson L, Gibson P. Methotrexate as a steroid sparing agent for asthma in adults. Cochrane Database Syst Rev 2000; (2): CD000391.
- 27. Grainge C, Jayasekera N, Dennison P, et al. Case series reporting the effectiveness of mycophenolate mofetil in treatment-resistant asthma. Eur Respir J 2013; 42: 1134-1137.
- 28. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659-668.
- 29. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA 2016; 315: 1715-1725.
- 30. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573-582.
- 31. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014; (1): CD003559.
- 32. Soler M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254-261.
- 33. Lieberman PL, Umetsu DT, Carrigan GJ, Rahmaoui A. Anaphylactic reactions associated with omalizumab administration: analysis of a case-control study. J Allergy Clin Immunol 2016; 138: 913-915.e2.
- 34. Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 2007; 176: 1062-1071.
- 35. Kips JC, O’Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 2003; 167: 1655-1659.
- 36. Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360: 985-993.
- 37. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651-659.
- 38. Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355-366.
- 39. Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125-1132.
- 40. Bachert C, Gevaert P, Hellings P. Biotherapeutics in chronic rhinosinusitis with and without nasal polyps. J Allergy Clin Immunol Pract 2017; 5: 1512-1516.
- 41. Yancey SW, Ortega HG, Keene ON, et al. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol 2017; 139: 1167-1175.e2.
- 42. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5: 390-400.
- 43. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189-1197.
- 44. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017; (9): CD010834.
- 45. Zhang XY, Simpson JL, Powell H, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy 2014; 44: 1137-1145.
- 46. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4: 549-556.
- 47. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115-2127.
- 48. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128-2141.
- 49. Nair P, Barker P, Goldman M. Glucocorticoid sparing of benralizumab in asthma. N Engl J Med 2017; 377: 1205.
- 50. Teva Pharmaceutical Industries. Teva announces top-line results from phase iii studies of subcutaneously administered reslizumab in patients with severe eosinophilic asthma [media release]. 22 Jan 2018. http://www.tevapharm.com/news/teva_announces_top_line_results_from_phase_iii_studies_of_subcutaneously_administered_reslizumab_in_patients_with_severe_eosinophilic_asthma_01_18.aspx (viewed May 2018).
- 51. Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31-44.
- 52. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287-2303.
- 53. Corren J, Parnes JR, Wang L, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377: 936-946.
- 54. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2016; 4: 699-707.
- 55. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181: 116-124.
- 56. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013; 132: 1295-1302.





Summary