The known Anticoagulation therapy reduces stroke risk in patients with atrial fibrillation/flutter and mortality among those with venous thromboembolism. Non-vitamin K antagonist oral anticoagulants (NOACs) are safe alternatives to warfarin therapy, which requires access to health care services not always available in rural areas.
The new About one-quarter of patients in rural Western Australia with a relevant indication were not prescribed anticoagulation therapy. Proximity of the patient to the treating hospital influenced the choice of therapy. Treating rural patients with NOACs was both convenient and safe.
=The implications NOACs may further improve anticoagulation therapy rates for patients living in remote locations.
Anticoagulation treatment reduces the incidence of ischaemic stroke in patients at risk of arterial thromboembolism and reduces mortality in patients with venous thromboembolism.1,2 Although effective, it also increases the propensity for bleeding and haemorrhagic complications in some patients.
Vitamin K antagonists such as warfarin have long been the mainstay of anticoagulation therapy. Their highly variable metabolism is influenced by food and drug interactions, genetic polymorphisms, age, and medical comorbidities.3 Individualised dose adjustment, guided by frequent laboratory testing of the international normalised ratio (INR) for prothrombin time, optimises the time in therapeutic range and minimises the risk of thromboembolic and haemorrhagic events.3 For warfarin therapy to be safe and effective, adequate access to health care services is therefore essential.
Twenty-nine per cent of Australians live in rural and remote areas where limited access to health care services is associated with higher mortality rates.4 Optimising warfarin therapy in this population can be challenging; while point-of-care testing may be helpful, it reduces neither the frequency of testing nor the frequency of visits to a general practitioner.5 Accordingly, remoteness in Australia is a major barrier to warfarin therapy.6
Non-vitamin K antagonist oral anticoagulants (NOACs) are an established therapeutic option for long term anticoagulation therapy, and are safer than warfarin in urban settings.7,8 Although frequent laboratory testing is not required, NOACs require satisfactory renal function and strict adherence (because of their shorter plasma half-lives), and there is also concern about their reversibility.9 Rural patients requiring long term anticoagulation therapy may benefit from the convenience of NOACs, particularly when warfarin therapy is logistically challenging.
We examined the use of different anticoagulation therapies in rural Western Australia, to establish whether remoteness from health care services affects the choice of anticoagulation therapy, and to gather preliminary data on the safety and efficacy of differing anticoagulation therapies in this region.
Methods
We retrospectively identified patients with indications for anticoagulation therapy in four rural hospitals in WA. Albany, Geraldton and Bunbury are classified by the Australian Rural, Remote and Metropolitan classification system10 as Small Rural Centres, while Kalgoorlie is a Remote Centre; they are respectively 413 km, 417 km, 174 km and 593 km from Perth, the metropolitan capital of WA and itself one of the most isolated cities in the world. Each site acts as a regional centre for the corresponding WA Country Health Service region; together these regions include more than two-thirds of the WA rural population.
Medical coding services identified admissions of patients for an overnight stay with a principal diagnosis of atrial fibrillation/flutter (AF; International Classification of Diseases, revision 10 [ICD-10] codes I48.x) or venous thromboembolism (VTE; includes pulmonary embolism and deep vein thrombosis: ICD-10 codes I26.x and I82.x) between 1 January 2014 and 31 December 2015. Discharge summaries for all hospital admissions before and including the index admission were reviewed for demographic data, results of relevant investigations, and medical comorbidities. Anticoagulation therapies were identified as warfarin, NOACs (dabigatran, apixaban, rivaroxaban) or low molecular weight heparin (LMWH), as prescribed prior to and at discharge from the index admission. Patients were excluded if insufficient medical or medication history was available in their index discharge summary, or if significant discrepancies between medications prescribed and medical comorbidities were identified. HAS-BLED bleeding risk scores11 were calculated for all patients, as were CHA2DS2–VASc stroke risk scores12 for patients with AF. Scores were calculated according to access to relevant laboratory investigations and information available in index and previous discharge summaries. A falls risk was identified if a patient had previously been admitted to hospital for complications of a fall, or if a falls risk was recorded in the index discharge summary. Distance from hospital of admission was estimated from postcodes and shortest road distances between two sites; patients living in the same postcode as their index hospital were deemed to live locally. Patients with no fixed address or from interstate were classified as “out of region”.
A patient-specific statewide medical record number was allocated to each patient before or during their index admission. Any patient with a subsequent admission (to October 2016) was identified in a statewide electronic medical record database by their medical record number, and corresponding discharge summaries were inspected for reason for admission, changes in anticoagulation therapy, and the outcomes of interest (safety and lack-of-efficacy events).
A safety event was defined as a hospital admission in WA for either a major bleeding event or a clinically relevant non-major bleeding event according to the International Society of Thrombosis and Haemostasis classification criteria.13,14 For the purpose of this study, patients with a severe INR elevation (greater than 9) requiring in-hospital intervention were classified as having clinically relevant non-major bleeding events, and patients with significant unexplained anaemia requiring transfusion of two or more units of blood products were classified as having major bleeding events. Lack-of-efficacy events were defined as re-admission to a public hospital in WA with a principal or secondary diagnosis of a new transient ischaemic attack, ischaemic stroke, systemic arterial thromboembolism, or VTE, as diagnosed by the treating team at the time of admission.
Data were analysed in SPSS Statistics 21 (IBM). Baseline characteristics are reported as frequencies, means (with standard deviations [SDs]), or medians (with interquartile ranges [IQRs]). The statistical significance of differences between categorical variables was assessed in χ2 tests, and of continuous variables in Mann–Whitney U tests. Non-local patients were grouped into tertiles of distance to their index hospital.
Ethics approval
This research was approved by the Western Australian Country Health Services, Human Research and Ethics Committee; a waiver of informed consent by patients was granted (reference, 2016/19).
Results
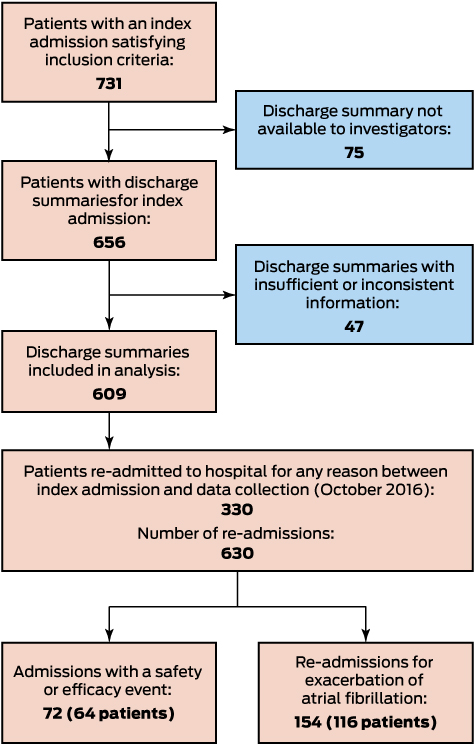
In total, 731 admitted patients with an indication for anticoagulation therapy were identified; a discharge summary was unavailable for 75 patients (10%), and information for a further 47 (6.4%) was insufficient or inconsistent. Discharge summaries for 609 patients were appropriate for inclusion (Box 1). The characteristics of excluded patients were similar to those of the study cohort (data not shown): their mean age was 67.2 years (SD, 15.0 years), 67 patients (55%) were men, and 93 (76%) had an index admission for AF.
Baseline characteristics
At discharge from the index admission, warfarin was prescribed as anticoagulant for 200 patients (32.8%), a NOAC for 205 (33.7%), and LMWH for 46 (7.6%); no anticoagulant was prescribed for 158 patients (25.9%). Larger proportions of patients prescribed warfarin than of those prescribed NOACs had diabetes (29% v 16%; P = 0.001) or chronic kidney disease (14% v 6%: P = 0.004); they also had a higher median CHA2DS2–VASc score (patients with AF only: 4 [IQR, 3–5] v 3 [IQR, 2–4]; P = 0.002) (Box 2). Of the NOACs, rivaroxaban was prescribed for 122 patients (59.5%), apixaban for 78 (38%), and dabigatran for five (2%). Most patients (55%) lived in the same postcode as the hospital of admission (Box 3).
Choice of anticoagulation therapy
Patients with AF receiving anticoagulation therapy were more often prescribed a NOAC than warfarin during the index admission (56.0% v 42.2% of those who received an anticoagulant; P < 0.001). Patients with VTE receiving anticoagulation therapy were more often prescribed warfarin than a NOAC (48% v 29% of those who received an anticoagulant; P < 0.001). Of the patients prescribed LMWH, 41 (89%) had VTE and five (11%) AF; the five patients with AF were discharged for outpatient management of anticoagulation (Box 3).
Of the 432 patients with AF, 372 (86.1%) had an indication for anticoagulation according to current guidelines (CHA2DS2–VASc ≥ 1 in men; ≥ 2 in women),15 of whom 257 (69.1%) were prescribed an anticoagulant. Of the 60 patients who did not meet guideline recommendations for anticoagulation therapy, 18 (30%) were also prescribed an anticoagulant. Box 4 depicts the prescription rates for each anticoagulant by CHA2DS2–VASc score. Of the 115 patients with an indication for anticoagulation therapy who were not prescribed an anticoagulant, 14 (12%) had a high bleeding risk (HAS-BLED score ≥ 3) and 17 (15%) had a falls risk; 84 (73%) had no obvious contraindications, and did not live further from the hospital than those prescribed anticoagulation therapy. Patients with AF who were not prescribed anticoagulation therapy were more likely than those who were to receive antiplatelet therapy (110 [70%] v 64 [23%]; P < 0.001).
Remoteness as a factor in selection of anticoagulation therapy
Warfarin was prescribed for 38% of patients who lived locally, a NOAC was prescribed for 31% (P = 0.013); for non-local patients, the respective proportions were 29% and 36% (P = 0.08). Patients from outside the region were more often prescribed a NOAC than warfarin (43% v 9%, P = 0.021) (Box 3).
Safety and lack-of-efficacy events
There were 72 safety and lack-of-efficacy events affecting 64 (10%) patients; eight patients (1%) experienced both safety and lack-of-efficacy events. Forty-three patients (7.1%) experienced safety events (Box 5). Compared with patients who did not experience safety events, patients with safety events were significantly older (mean, 74.6 years [SD, 13.3] v 66.2 years [SD, 15.0]; P < 0.001), and had higher median CHA2DS2–VASc (4 [IQR 3–5] v 3 [IQR, 1–4]; P < 0.001) and HAS-BLED scores (2 [IQR, 1–2] v 1 [IQR, 1–2]; P = 0.001).
Safety events were more common among patients treated with warfarin than those treated with a NOAC (10% v 4%; P = 0.027) or those who did not receive anticoagulation therapy (4%; P = 0.040) (Box 5).
Twenty-nine patients (4.8%) were hospitalised because of lack-of-efficacy events (Box 6). These patients had a higher median CHA2DS2–VASc score (4 [IQR, 3.5–5] v 3 [IQR, 2–4]; P = 0.005) than patients without such events, but their ages (mean, 68.5 years [SD, 17.0] v 66.7 years [SD, 15.0]; P = 0.27) and median HAS-BLED scores (1 [IQR, 1–2] v 1 [IQR, 0–2]; P = 0.65) were similar.
Discussion
This is the first study since the introduction of NOACs to evaluate the choice of anticoagulation therapy for Australians with AF living in rural areas. We found that 31% of patients with AF and a clear indication for therapy were not prescribed anticoagulation therapy.15 While the rate of prescription was low, an earlier Australian study of 241 women aged 75 or more with AF reported an anticoagulation rate in rural areas of 51%.16 Our rate was also higher than the 27% reported from a study of 19 613 Australians treated for AF at tertiary hospitals between 1999 and 2012,17 suggesting that anticoagulation treatment rates in rural Australia have improved in recent times.
In our study, only a small proportion of patients with AF not prescribed anticoagulation therapy had a relevant contraindication, such as a high HAS-BLED score or falls risk. Interestingly, neither of these factors should preclude anticoagulation therapy in patients with AF who are at risk of stroke.15 Most patients not prescribed anticoagulation therapy received antiplatelet treatment, but aspirin is not effective in preventing stroke in patients with AF.18 The introduction of NOACs has probably played a role in improving anticoagulation rates. However, the transfer of evidence-based knowledge into rural clinical settings remains difficult, and further strategies are required to overcome this problem.
Remoteness as a factor in the prescribing of anticoagulation therapy
Warfarin was prescribed more frequently than NOACs for local patients; for patients located more than 3 km from the treating hospital, NOACs were prescribed more frequently than warfarin, but the difference was not statistically significant. These results suggest that remoteness influences the choice of anticoagulation therapy. A study of 597 patients with AF in Missouri found that rural patients were significantly less likely to receive anticoagulation therapy than urban patients.19 In contrast, a Canadian study of 83 513 patients with AF found no difference between anticoagulation prescription rates for rural and urban patients.20 Although there are significant differences between rural Australians and Canadians in cardiovascular health and health care services,21 the Australian study of 241 rural and urban women with AF found no significant difference in prescription rates.16 Improving anticoagulation therapy rates for people with AF provides the greatest opportunity for reducing the incidence of ischaemic stroke in rural Australia.22
Safety and efficacy outcomes
Warfarin was associated with higher rates of safety events than NOACs, and with similar rates of lack-of-efficacy events. This difference in the frequency of safety events was not reflected by a difference between the anticoagulation groups in HAS-BLED scores; there was, however, a significant difference in median CHA2DS2–VASc score, which is also a predictor of bleeding risk.23 The high rates of bleeding in patients receiving LMWH (Box 4) is difficult to explain, but may be connected with comorbidities not examined in our study. Increasing evidence for the safety of NOACs, coupled with the development of reversal agents, might increase their use, and consequently improve anticoagulation rates for rural patients with AF.
Choice of anticoagulation therapy
NOACs were the most commonly prescribed anticoagulation therapy for patients with AF, suggesting good uptake in rural WA. A study of 5442 Australian veterans prescribed anticoagulation for AF for the first time found that 72% were prescribed NOACs and 28% warfarin.24 The uptake of NOACs in rural areas may, however, be slower. A prospective study of 342 rural patients in Greece found that 21% of all anticoagulant prescriptions for patients with AF at risk of stroke were for NOACs.25 We found a preference for prescribing NOAC therapy for patients with AF, and this was associated with improved anticoagulation rates that may subsequently result in a cost benefit to the health care system through reduced stroke rates. For remote patients, the lower need for monitoring when using NOACs may also provide a personal economic benefit.
We found that warfarin was prescribed more frequently than NOACs for patients with VTE, suggesting a slower uptake of NOACs for this indication, perhaps because VTE is a more recent indication for NOAC therapy.
Limitations of our study
The retrospective design of our study is the major limitation. Medical coding services probably allow capture of all patients hospitalised because of AF or VTE in the regions we examined, but about 17% of patients were excluded because discharge summaries were unavailable, incomplete, or inconsistent. The ability to identify safety and lack-of-efficacy outcomes was limited to re-hospitalisations, and mortality data outside the hospital setting were not accessible. Most major bleeding events in rural WA result in hospital admission, and the higher rates of comorbidities among patients receiving warfarin mean that unidentified deaths may be more likely in this group. About one-third of rural WA was not represented in our data, perhaps limiting the generalisability of our findings.
Conclusion
NOACs were prescribed for about one-third of patients in rural WA with an indication for anticoagulation therapy. About one-third of patients with AF and with an indication for anticoagulation therapy (at risk of stroke according to their CHA2DS2–VASc score) received no anticoagulant therapy. Prescribing NOACs may improve rates of anticoagulation therapy in rural areas where the access to health care services required by warfarin therapy is limited.
Box 2 – Demographic and clinical characteristics of patients receiving anticoagulation therapy, by anticoagulant
|
|
All patients |
Anticoagulation therapy |
|||||||||||||
|
Warfarin |
NOACs |
LMWH |
None |
||||||||||||
|
|
|||||||||||||||
|
Number of patients |
609 |
200 (32.8%) |
205 (33.7%) |
46 (7.6%) |
158 (25.9%) |
||||||||||
|
Age (years), mean (SD) |
66.8 (15.1) |
67.3 (14.6) |
66.9 (15.4) |
64.1 (13.0) |
66.8 (5.9) |
||||||||||
|
Sex (men) |
305 (50%) |
93 (46%) |
107 (52%) |
24 (52%) |
81 (51%) |
||||||||||
|
Comorbidities |
|
|
|
|
|
||||||||||
|
Falls risk |
42 (6.9%) |
12 (6.0%) |
9 (4%) |
1 (2%) |
20 (13%) |
||||||||||
|
Chronic kidney disease |
61 (10%) |
29 (14%) |
12 (6%) |
3 (6%) |
17 (11%) |
||||||||||
|
Congestive heart failure |
113 (18.6%) |
48 (24%) |
48 (23%) |
1 (2%) |
16 (10%) |
||||||||||
|
Hypertension |
360 (59.1%) |
132 (66.0%) |
124 (60.5%) |
17 (37%) |
87 (55%) |
||||||||||
|
Diabetes mellitus |
119 (19.5%) |
58 (29%) |
32 (16%) |
6 (13%) |
23 (15%) |
||||||||||
|
Previous stroke or transient ischaemic attack |
50 (8.2%) |
26 (13%) |
16 (7.8%) |
1 (2%) |
7 (4%) |
||||||||||
|
Previous venous thromboembolism |
18 (3.0%) |
13 (6.5%) |
2 (1%) |
3 (6%) |
0 |
||||||||||
|
Vascular disease |
129 (21.2%) |
50 (25%) |
40 (20%) |
6 (13%) |
33 (21%) |
||||||||||
|
Antiplatelet therapy |
195 (32.0%) |
42 (21%) |
35 (17%) |
7 (15%) |
111 (70.2%) |
||||||||||
|
Risk scores |
|
|
|
|
|
||||||||||
|
HAS-BLED, median (IQR) |
1 (1–2) |
1 (0–2) |
1 (1–1) |
1 (0–2) |
2 (1–2) |
||||||||||
|
CHA2DS2–VASc, median (IQR) |
3 (2–4) |
4 (3–5) |
3 (2–4) |
1 (1–2) |
2 (1–4) |
||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; LMWH = low molecular weight heparin; NOACs = non-vitamin K oral anticoagulants; SD = standard deviation. All percentages are column percentages. |
|||||||||||||||
Box 3 – Choice of anticoagulation therapy, by location and indication for therapy
|
|
All patients |
Anticoagulation therapy |
|||||||||||||
|
Warfarin |
NOACs |
LMWH |
None |
||||||||||||
|
|
|||||||||||||||
|
Number of patients |
609 |
200 (32.8%) |
205 (33.7%) |
46 (7.6%) |
158 (25.9%) |
||||||||||
|
Distance between patients’ residence and hospital of admission |
|||||||||||||||
|
Local patients |
335 (55.0%) |
126 (38%) |
104 (31%) |
20 (6%) |
85 (25%) |
||||||||||
|
Non-local patients |
251 (45.0%) |
72 (29%) |
91 (36%) |
24 (10%) |
64 (25%) |
||||||||||
|
Non-local, ≤ 30 km |
85 (14%) |
25 (29%) |
38 (45%) |
6 (7%) |
16 (19%) |
||||||||||
|
Non-local, >30 km and < 70 km |
79 (13%) |
24 (30%) |
29 (37%) |
7 (9%) |
19 (24%) |
||||||||||
|
Non-local, ≥ 70 km |
87 (14%) |
23 (26%) |
24 (28%) |
11 (13%) |
29 (33%) |
||||||||||
|
Out of region |
23 (4%) |
2 (9%) |
10 (43%) |
2 (9%) |
9 (39%) |
||||||||||
|
Indication for anticoagulation therapy |
|||||||||||||||
|
Atrial fibrillation/flutter |
432 (70.9%) |
116 (26.9%) |
154 (35.6%) |
5 (1%) |
157 (36.3%) |
||||||||||
|
Pre-existing diagnosis (therapy prescribed on discharge from index admission) |
220 (36.1%) |
65 (27%) |
84 (38%) |
1 (0.4%) |
70 (32%) |
||||||||||
|
New diagnosis |
212 (34.8%) |
51 (24%) |
70 (33%) |
4 (4%) |
87 (41%) |
||||||||||
|
Venous thromboembolism |
177 (29.1%) |
84 (47%) |
51 (29%) |
41 (23%) |
1 (0.6%) |
||||||||||
|
|
|||||||||||||||
|
LMWH = low molecular weight heparin; NOACs = non-vitamin K oral anticoagulants. Except for “All patients” column, all percentages are row percentages. |
|||||||||||||||
Box 4 – Numbers of patients receiving each anticoagulation therapy type, by CHA2DS2–VASc score
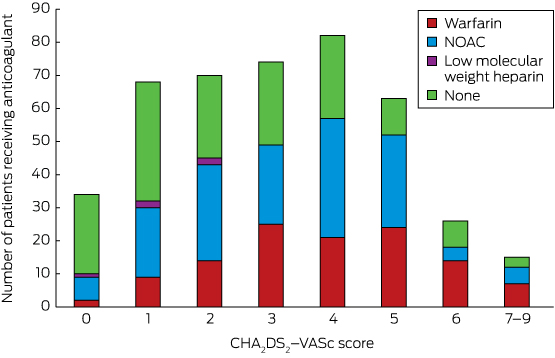
NOACs = non-vitamin K oral anticoagulants.
Box 5 – Patients with safety (bleeding) events, by anticoagulation therapy group
|
|
Anticoagulation therapy* |
||||||||||||||
|
Warfarin |
NOACs |
LMWH |
None |
||||||||||||
|
|
|||||||||||||||
|
Total number of patients |
197 |
204 |
48 |
160 |
|||||||||||
|
Patients with an event |
20 (10%) |
9 (4%) |
7 (15%) |
7 (4%) |
|||||||||||
|
Major bleeding events |
13 (65%) |
7 (78%) |
4 (57%) |
6 (86%) |
|||||||||||
|
Clinically relevant, non-major bleeding events |
7 (35%) |
2 (22%) |
3 (43%) |
1 (14%) |
|||||||||||
|
|
|||||||||||||||
|
LMWH = low molecular weight heparin; NOACs = non-vitamin K oral anticoagulants. * Changes from in the numbers of patients in each group reflect the number of patients for whom anticoagulation therapy changed between the index admission and re-admission with the safety event. Therapy was changed between the index admission and an outcome of interest for nine patients (2%) who experienced 11 safety or lack-of-efficacy events. Anticoagulation therapy was changed for two patients (0.3%) between safety and lack-of-efficacy events (patients who had a change in anticoagulation therapy after the index admission but prior to re-admission with an outcome of interest). |
|||||||||||||||
Box 6 – Patients with lack-of-efficacy (thromboembolic) events, by anticoagulation therapy group
|
|
Anticoagulation therapy |
||||||||||||||
|
Warfarin |
NOACs |
LMWH |
None |
||||||||||||
|
|
|||||||||||||||
|
Number of patients* |
196 |
207 |
47 |
159 |
|||||||||||
|
Patients with an event |
8 (4%) |
10 (5%) |
4 (8%) |
7 (4%) |
|||||||||||
|
Ischaemic stroke/transient ischaemic attack |
4 (50%) |
7 (70%) |
0 |
5 (71%) |
|||||||||||
|
Recurrent venous thromboembolism |
4 (50%) |
3 (30%) |
4 (100%) |
2 (29%) |
|||||||||||
|
|
|||||||||||||||
|
LMWH = low molecular weight heparin; NOACs = non-vitamin K oral anticoagulants. * Changes from in the numbers of patients in each group reflect the number of patients for whom anticoagulation therapy changed between the index admission and re-admission with the lack-of-efficacy event. |
|||||||||||||||
Received 10 February 2017, accepted 2 May 2017
- Jamie W Bellinge1
- Jarrad J Paul1
- Liam S Walsh2
- Lokesh Garg3
- Gerald F Watts4
- Carl Schultz4
- 1 Royal Perth Hospital, Perth, WA
- 2 Bunbury Regional Hospital, Bunbury, WA
- 3 South West Health Campus, Bunbury, WA
- 4 University of Western Australia, Perth, WA
No relevant disclosures.
- 1. Barrit DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet 1960; 1: 1309-1312.
- 2. Stroke Prevention in Atrial Fibrillation Investigators. Stroke Prevention in Atrial Fibrillation Study: final results. Circulation 1991; 84: 527-539.
- 3. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133: 60S-198S.
- 4. Australian Institute of Health and Welfare. Australia’s health 2016 (AIHW Cat. No. AUS 199; Australia’s Health Series No. 15). Canberra: AIHW, 2016.
- 5. Laurence C, Gialamas A, Yelland L, et al. Point of Care Testing in General Practice Trial: final report. Jan 2009. http://www.appn.net.au/Data/Sites/1/SharedFiles/Publications/200901-poctfinalreport27jan09amended5feb09.pdf (viewed Dec 2016).
- 6. Peterson GM, Boom K, Jackson SL, Vial JH. Doctors’ beliefs on the use of antithrombotic therapy in atrial fibrillation: identifying barriers to stroke prevention. Intern Med J 2002; 32: 15-23.
- 7. Hicks T, Stewart F, Eisinga A. NOACs versus warfarin for stroke prevention in patients with AF: a systematic review and meta-analysis. Open Heart 2016; 3: e000279.
- 8. Van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124: 1968-1975.
- 9. Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program 2013; 2013: 464-470.
- 10. Australian Institute of Health and Welfare. Rural regional and remote health: a guide to remoteness classifications (AIHW Cat. No. PHE 53). Canberra: AIHW, 2004. Archived: https://web.archive.org/web/20170712025537/http://www.aihw.gov.au/publication-detail/?id=6442467589 (viewed Dec 2016).
- 11. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011; 57: 173-180.
- 12. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137: 263-272.
- 13. Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015; 13: 2119-2126.
- 14. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 692-694.
- 15. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893-2962.
- 16. Jordan S, Wilson A, Dobson A. Management of heart conditions in older rural and urban Australian women. Intern Med J 2011; 41: 722-729.
- 17. Wong CX, Lee SW, Gan SW, et al. Underuse and overuse of anticoagulation for atrial fibrillation: a study in Indigenous and non-Indigenous Australians. Int J Cardiol 2015; 191: 20-24.
- 18. Själander S, Själander A, Svensson PJ, Friberg L. Atrial fibrillation patients do not benefit from acetylsalicylic acid. Europace 2014; 16: 631-638.
- 19. Flaker GC, McGowan DJ, Boechler M, et al. Underutilization of antithrombotic therapy in elderly rural patients with atrial fibrillation. Am Hear J 1999; 137: 307-312.
- 20. Avgil Tsadok M, Jackevicius CA, Essebag V, et al. Warfarin treatment and outcomes of patients with atrial fibrillation in rural and urban settings. J Rural Health 2015; 31: 310-315.
- 21. Pong RW, DesMeules M, Lagacé C. Rural-urban disparities in health: How does Canada fare and how does Canada compare with Australia? Aust J Rural Health 2009; 17: 58-64.
- 22. Newbury J, Kleinig T, Leyden J, et al. Stroke Epidemiology in an Australian Rural Cohort (SEARCH). Int J Stroke 2017; 12: 161-168.
- 23. Roldán V, Marín F, Manzano-Fernández S, et al. The HAS-BLED Score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2–VASc Scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2013; 62: 2199-2204.
- 24. Pratt NL, Ramsay EN, Caughey GE, et al. Uptake of novel oral anticoagulants in Australia. Med J Aust 2016; 204: 104-105. <MJA full text>
- 25. Papakonstantinou PE, Asimakopoulou NI, Gargeraki A, et al. Anticoagulation management in non-valvular atrial fibrillation in rural and remote Crete. A single-center study from the region of Sitia. Hellenic J Cardiol 2016; 57: 279-281.





Abstract
Objective: To determine the use of different anticoagulation therapies in rural Western Australia; to establish whether remoteness from health care services affects the choice of anticoagulation therapy; to gather preliminary data on anticoagulation therapy safety and efficacy.
Design: Retrospective cohort study of patients hospitalised with a principal diagnosis of atrial fibrillation/flutter (AF) or venous thromboembolism (VTE) during 2014–2015.
Setting: Four hospitals serving two-thirds of the rural population of Western Australia.
Participants: 609 patients with an indication for anticoagulation therapy recorded in their hospital discharge summary for index admission.
Main outcome measures: Prescribing rates of anticoagulation therapies by indication for anticoagulation and distance of patient residence from their hospital. The primary safety outcome was re-hospitalisation with a major or clinically relevant non-major bleeding event; the primary lack-of-efficacy outcome was re-hospitalisation for a thromboembolic event.
Results: The overall rates of prescription of NOACs and warfarin were similar (34% v 33%). A NOAC was prescribed more often than warfarin for patients with AF (56.0% v 42.2% of those who received an anticoagulant; P < 0.001), but less often for patients with VTE (29% v 48%; P < 0.001). Warfarin was prescribed for 38% of patients who lived locally, a NOAC for 31% (P = 0.013); for non-local patients, the respective proportions were 29% and 36% (P = 0.08). 69% of patients with AF and a CHA2DS2–VASc score ≥ 1 were prescribed anticoagulation therapy. Patients treated with NOACs had fewer bleeding events than patients treated with warfarin (nine events [4%] v 20 events [10%]; P = 0.027).
Conclusions: In rural WA, about one-third of patients with an indication for anticoagulation therapy receive NOACs, but one-third of patients with AF and at risk of stroke received no anticoagulant therapy, and may benefit from NOAC therapy.