Clinical trials have shown that novel oral anticoagulants (NOACs) are as efficacious as warfarin in reducing the risk of stroke in patients with atrial fibrillation.1-3 However, concerns regarding efficacy and safety in real-world populations remain, particularly in patients aged 75 years and older and those with comorbid conditions.4 Three NOACs — dabigatran, rivaroxaban and apixaban — were listed by the Pharmaceutical Benefits Scheme in 2013, but there is little information about their uptake in clinical practice in Australia.
We conducted a retrospective observational study to examine overall use and initiation of oral anticoagulants, and the characteristics of patients for whom they are prescribed. We used administrative claims data from the Australian Government Department of Veterans’ Affairs for patients who were full entitlement holders (ethics approval E010/010). Monthly rates of NOAC and warfarin use were calculated for the period 1 January 2012 – 31 August 2014, and numbers of veterans initiated each month on low-dose and high-dose NOACs were calculated for the period 1 September 2013 – 31 August 2014. Clinical characteristics of new users of NOACs or warfarin (no use of warfarin in the previous 12 months) were compared using χ2 or Wilcoxon rank sum tests.
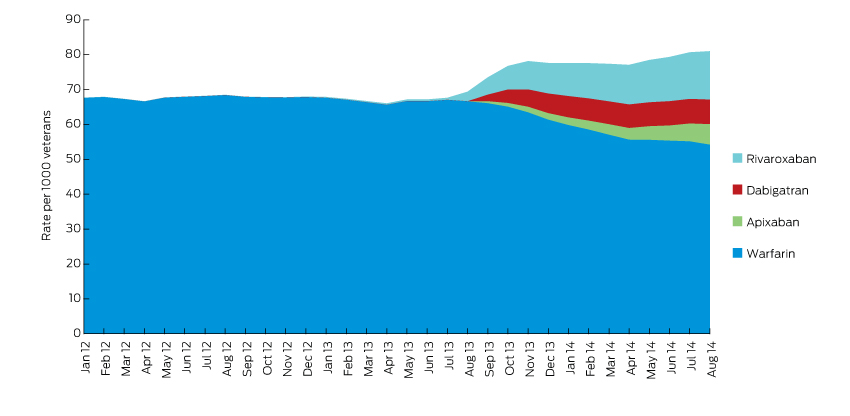
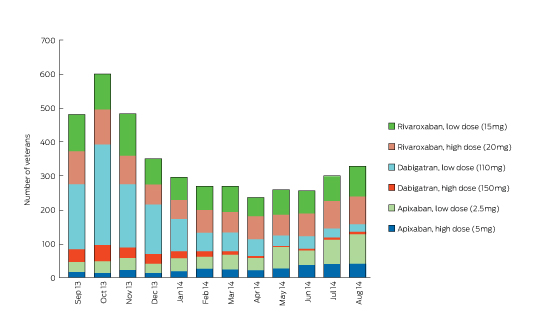
In the year after NOACs were listed on the Pharmaceutical Benefits Scheme, the overall rate of anticoagulation therapy increased, while the rate of warfarin use decreased (Box 1). There were 3936 and 1506 new users of NOACs and warfarin respectively. The median age of patients in the cohort was 86 years. Use of low-dose formulations of NOACs was most common, potentially reflecting impaired renal function in this older population (Box 2).
Those initiated on NOACs were younger, more likely to be men, had fewer comorbidities, and were less likely to reside in aged care facilities. Use of dose administration aids was similar for NOAC and warfarin users (10.6% [416/3936] and 10.7% [161/1506] respectively). Those initiated on NOACs had fewer hospitalisations recorded in the previous 12 months for gastrointestinal bleed, stroke and myocardial infarct compared with those initiated on warfarin (1.7% [67/3936] v 3.1% [46/1506], P = 0.0017; 3.3% [128/3936] v 7.2% [108/1506], P < 0.0001; and 2.2% [86/3936] v 4.5% [68/1506], P < 0.0001 respectively). Concomitant use of antithrombotic medicines was significantly lower in those initiated on NOACs compared with those initiated on warfarin (21.0% [803/3819] v 35.1% [491/1397], P < 0.0001). Non-steroidal anti-inflammatory drug use was greater in those initiated on NOACs (8.7% [332/3819] v 6.2% [87/1397], P < 0.004). (The denominators 3819 and 1397 include only those who survived 120 days after initiation of the NOAC or warfarin.) Together with the lack of reversibility of the anticoagulation effect of NOACs, these results are of concern and suggest that prescribers should consider the potential risk of bleeds when NOACs are co-administered with medicines such as non-steroidal anti-inflammatory drugs. This analysis was performed in an older veteran population and may not be generalisable to the wider Australian population.
The overall increased use of oral anticoagulants since the introduction of NOACs may reflect use in patients who were previously considered unsuitable for treatment with warfarin. It appears that pre-existing risk factors are being considered by prescribers when making the choice between NOACs and warfarin. However, prescribers need to remain vigilant to the risk of bleeding with NOACs, particularly in patients who are taking other medicines that might increase bleeding risk.
Received 3 September 2015, accepted 26 November 2015
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation [Erratum appears in N Engl J Med 2010; 363: 1877]. N Engl J Med 2009; 361: 1139-1151.
- 2. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883-891.
- 3. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981-992.
- 4. Vaughan MS, Rose A. Safety of new oral anticoagulants. BMJ 2015; 350: h1679.





This work was supported by a National Health and Medical Research Council Centre of Research Excellence in Post-Market Surveillance of Medicines and Medical Devices grant (grant number APP1040938). Nicole Pratt is supported by a National Health and Medical Research Council Early Career Fellowship (grant number GNT1035889).
No relevant disclosures.