The known Primary cutaneous melanomas may arise de novo or in association with a pre-existing naevus. Understanding the initial presentation of superficial spreading and nodular melanoma subtypes is vital for facilitating their early detection.
The new Most melanomas develop without a pre-existing naevus, particularly nodular melanomas, melanomas in patients over 70 years of age, and amelanotic/hypomelanotic melanomas.
The implications It is important for public health campaigns to emphasise the importance of detecting suspicious de novo lesions, as well as changing lesions.
Although only 10–15% of all invasive melanomas are nodular melanomas, this subtype is the predominant contributor to melanoma-related deaths.1 Nodular melanomas often elude early detection and account for a disproportionately high number of thick primary melanomas, partly because of their atypical clinical presentation (Box 1).1-3 Superficial spreading melanoma is the most common melanoma subtype, accounting for 55–65% of all melanomas (Box 2).1
Nodular melanomas often do not conform to the classical ABCDE criteria used to alert doctors and patients to the possibility of superficial spreading melanoma.1,3,4 In contrast to superficial spreading melanoma, characterised by a radial growth phase followed by invasion into the dermis, nodular melanoma is characterised by early vertical growth. Nodular melanoma is also associated with more rapid vertical growth,5 so that improving diagnostic accuracy of nodular melanoma is crucial.1 Understanding the initial presentation of both these melanoma subtypes is vital for their early detection.
Primary cutaneous melanomas may arise de novo or in association with a pre-existing naevus. Based on histological assessment, naevus-associated melanomas are estimated to represent 19–58% of all cutaneous melanomas.6-17 A clinical history of a longstanding precursor pigmented lesion is reported by patients for 40–45% of all biopsy-proven melanomas.11 However, a reported precursor pigmented lesion may be a slowly progressing early melanoma or a benign naevus, so that this information is not always reliable.16 Indeed, it has been reported that about one-third of patients without histological evidence of a naevus erroneously recall a pre-existing naevus.18
A correlation between various clinical and pathological characteristics and naevus-associated and de novo melanoma has been reported in several studies.6,9,12,14-17,19 Earlier studies have reported that nodular melanomas typically arise de novo and are only infrequently associated with a contiguous naevus.6,12,15 The aim of our study was to determine the frequency of naevus-associated melanoma among superficial spreading and nodular melanoma subtypes, which together account for about 70% of invasive melanomas and are the most common radial and vertical growth phase melanoma subtypes. Accurately understanding how often superficial spreading and nodular melanoma subtypes present as new or changing lesions may inform advice on how to detect these melanoma subtypes at their earliest presentation. Our secondary aim was to investigate the association between naevus-associated melanoma and other clinical and pathological characteristics, allowing us to determine whether there are any confounders of the potential relationship between naevus association and superficial spreading and nodular melanoma subtypes.
Methods
The Victorian Melanoma Service (VMS) is a statewide, multidisciplinary tertiary referral service based at the Alfred Hospital in Melbourne; it reviews about one-quarter of new melanoma cases in Victoria. The VMS database includes data on all patients treated at the service. Data for all patients with nodular melanomas and superficial spreading melanomas diagnosed at the VMS during 1994–2015 were included in this study.
An expert VMS dermatopathologist provided histopathological review of each melanoma. Tumour histological subtype was classified according to the World Health Organization classification system as superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, acral lentiginous melanoma, or desmoplastic melanoma. Our study assessed only the superficial spreading and nodular melanoma subtypes. The histopathological review recorded the presence or absence of an associated naevus for each melanoma. Data for patients were excluded from our study if information on the presence or absence of an associated naevus was not recorded.
The anatomic location of the primary tumour was categorised into the following groups: head and neck, trunk, and extremities. Patient-related variables were recorded by the treating doctor during the patients’ initial presentation.
Clinical and pathological characteristics extracted from the database included age, sex, Fitzpatrick skin type, personal history of melanoma, family history of melanoma, anatomic location of the primary tumour, Breslow thickness, histological subtype and amelanosis/hypomelanosis as assessed by the patient and family. Amelanosis/hypomelanosis describes melanomas with no or little pigment apparent to visual inspection.20
For statistical analysis, Breslow thickness was analysed as a continuous variable. Age was classified into ordinal groups (< 30, 30–49, 50–69, ≥ 70 years). Clinical and pathological characteristics of naevus-associated melanomas and de novo melanomas were compared in χ2 tests, and in univariable and multivariable logistic regression analyses; P < 0.05 was defined as statistically significant. Effect modification was assessed by calculating the strata-specific association between the proportion of naevus-associated melanoma and superficial spreading and nodular subtypes for multiple covariates (Breslow thickness, age, sex, amelanosis/hypomelanosis). All statistical analyses were performed in Stata 13 (StataCorp).
Ethics approval
Institutional ethics approval to conduct a cross-sectional study based on data from the prospectively maintained database at the VMS was granted by the Alfred Hospital (project number, 38/16).
Results
The study included 3678 primary cutaneous melanomas, of which 3260 were invasive and 418 in situ melanomas; 3057 primary melanomas (83.1%) were classified as superficial spreading melanoma (including all in situ tumours) and 621 (16.9%) as nodular melanoma. The median age of the patients was 54 years (interquartile range, 42–66 years); 53% were men. Of the 3678 melanomas, 1360 (37.0%) were histologically associated with a naevus, and 2318 (63.0%) were de novo; 71 of 621 nodular melanomas (11%) and 1289 of 3057 superficial spreading melanomas (42.2%) were histologically associated with a naevus. The clinical and pathological characteristics of naevus-associated and de novo melanoma are summarised in Box 3.
Univariable analysis
Thicker melanomas were less likely to be associated with a naevus (odds ratio [OR], 0.62 per mm increase in Breslow thickness; 95% confidence interval [CI], 0.58–0.67; P < 0.001). Melanomas on the trunk (OR, 3.28; 95% CI, 2.59–4.15; P < 0.001) and extremities (OR 1.51; 95% CI, 1.20–1.92; P = 0.001) were significantly more likely to be associated with a naevus than head and neck melanomas (Box 4).
The odds of a melanoma being naevus-associated declined with age (v patients under 30: 30–49 years, OR, 0.69; 95% CI, 0.53–0.91; P = 0.008; 50–69 years, OR, 0.45; 95% CI, 0.34–0.59; P < 0.001; 70 years or older, OR, 0.24; 95% CI, 0.18–0.33; P < 0.001). The odds of amelanotic/hypomelanotic melanomas being associated with a naevus were 70% lower than for pigmented melanomas (OR, 0.30; 95% CI, 0.22–0.41; P < 0.001) (Box 4).
Multivariable analysis
After adjusting for Breslow thickness, amelanosis/hypomelanosis, anatomic location, age, sex, personal and family history of melanoma, and Fitzpatrick skin type, the odds of being associated with a naevus were 3.05 times as high for superficial spreading melanomas as for nodular melanomas (OR, 3.05; 95% CI, 2.24–4.17, P < 0.001) (Box 4).
In the multivariable model, the inverse relationship between melanoma being naevus-associated and patient age remained (v patients under 30: 30–49 years, OR, 0.63; 95% CI, 0.46–0.86; P = 0.003; 50–69 years, OR, 0.42; 95% CI, 0.31–0.57; P < 0.001; 70 years or older, OR, 0.28; 95% CI, 0.20–0.40; P < 0.001). The odds of a melanoma being associated with a naevus declined by 25% for each 1 mm increase in Breslow thickness (OR, 0.75 per mm increase; 95% CI, 0.69–0.81; P < 0.001) (Box 4).
The relationship between anatomic location of the primary tumour on the trunk and being associated with a naevus was confirmed in the multivariable model: the odds of being associated with a naevus was 2.27 times higher for trunk melanomas than for melanomas on the head and neck region (OR, 2.27; 95% CI, 1.73–2.96; P < 0.001). Further, the odds of being associated with a naevus were 32% lower for amelanotic/hypomelanotic melanomas than for pigmented melanomas (OR, 0.68; 95% CI, 0.48–0.97; P = 0.035) (Box 4).
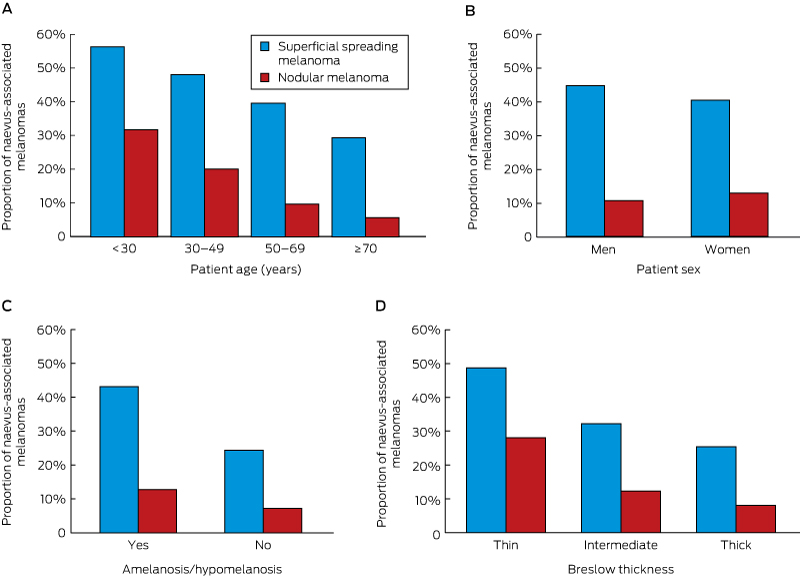
The strata-specific association between the proportion of naevus-associated melanomas and age group was the same for superficial spreading and nodular melanomas (P = 0.88), suggesting no effect modification by histologic subtype (Box 5, A). Further, there was no effect modification by sex (P = 0.27), amelanotic/hypomelanotic status (P = 0.79), and Breslow thickness (P = 0.96) (Box 5, B-D).
Discussion
In this study, we investigated the frequencies of naevus-associated melanoma among superficial spreading and nodular melanoma subtypes in a large and well characterised Australian cohort, and also examined clinical and pathological characteristics of naevus-associated melanomas. We found that 11.4% of nodular melanomas and 42.2% of superficial spreading melanomas were associated with a pre-existing naevus. After controlling for known confounders, nodular melanomas were significantly less likely than superficial spreading melanomas to be histologically associated with a naevus.
Previous studies have also found that nodular melanomas are less likely than other subtypes to be associated with a pre-existing naevus.6,9,12,16,17,19 A recent cross-sectional study in Brazil found that 8.7% of nodular melanomas were associated with a benign naevus.14 Similarly, a retrospective analysis in the United States identified that 35.4% of superficial spreading and 11.3% of nodular melanomas were naevus-associated,9 while an earlier study in Victoria found that only 2.7% of nodular melanomas were histologically associated with a pre-existing naevus.7
We also determined that the odds of an amelanotic/hypomelanotic melanoma being associated with a naevus were 32% lower than for pigmented melanomas. Amelanotic/hypomelanotic melanomas have been associated with the nodular subtype, head and neck location, and increased Breslow thickness.21-24 After controlling for potentially confounding covariates, we found an independent association between amelanotic/hypomelanotic melanoma and de novo development. This association has been reported by only one previous study: Thomas and colleagues21 compared the clinico-pathological features and survival of amelanotic and pigmented melanomas, and were the first to find a relationship between amelanosis and the absence of a pre-existing naevus (OR, 0.6; 95% CI, 0.4–1.0; P = 0.03).21 Their study assessed amelanosis histologically, while we assessed reports by patients and their families. Our study therefore contributes to the limited literature on an independent relationship between amelanosis/hypomelanosis and the absence of a pre-existing naevus.
We found that the odds of a melanoma being with associated with a naevus declined with increasing tumour thickness. It is possible that thick melanomas obliterate pre-existing naevus cells, and this contributes to the inverse relationship between the Breslow thickness of a melanoma and its being associated with a naevus. In fact, the odds ratios from the multivariate analysis indicated a weaker associations between naevus-associated melanoma and Breslow thickness and histological subtype than in the univariate analysis, which suggests that these covariates are mutually confounded. The inverse relationship between Breslow thickness and the presence of a pre-existing naevus remained in the multivariate analysis; this indicates that Breslow thickness was independently and inversely associated with naevus-associated melanoma, and that this relationship was not completely explained by the difference between nodular and superficial spreading melanoma subtypes.
An inverse relationship between tumour thickness and the presence of an associated pre-existing naevus has been reported in previous studies: Shitara and colleagues14 found that the median Breslow thickness of naevus-associated melanomas was significantly lower than for de novo melanomas, while Sagebiel10 reported that 65% of nodular and superficial spreading melanomas less than 1.69 mm thick, 46% of those 1.70–3.60 mm thick, and 32% of tumours more than 3.60 mm thick were histopathologically associated with benign naevus cells. Nonetheless, a recent prospective, single centre, observational study found that naevus-associated melanomas were more frequently associated with in situ tumours than invasive tumours; for invasive melanomas, however, being associated with a naevus was not related to tumour thickness.16
There is accumulating evidence for the aetiological heterogeneity of de novo naevus-associated melanomas.12,25 Whitehead and colleagues proposed a divergent pathway for cutaneous melanoma, premised on the propensity of the host to develop naevi.25,26 The first pathway describes naevus-prone individuals, in whom melanomas often arise in association with naevi, and require only modest amounts of sun exposure, after which tumour progression is predominantly driven by host factors. The second pathway describes individuals with a low propensity for developing naevi; these individuals are more likely to have de novo melanomas, and melanoma development requires a high degree of cumulative sun exposure.25,26
Consistent with this hypothesis, earlier observational studies found an association between naevus-associated melanoma and anatomic sites of intermittent sun exposure, such as the trunk.6,9,12,14-16,19 Accordingly, melanomas on sites associated with cumulative sun exposure, such as the head and neck, are less likely to be associated with a pre-existing naevus.6,9,12,14,25 This is consistent with our results, as the odds of a trunk melanoma being associated with a naevus were 2.27 times as high as for melanomas on the head and neck. Further, we found that naevus-associated melanomas were more common in younger patients, consistent with one previous study,9 although others have not found this association.12,16
Limitations of our study include likely referral bias, as the VMS is a statewide tertiary referral service to which patients with more advanced or aggressive disease are more likely to have been referred. A further limitation is that it may be difficult to histologically recognise the presence of a naevus in association with a melanoma, particularly dysplastic naevi and naevoid melanomas, despite the review of all melanomas by a dermatopathologist. The possibility of a fortuitous collision event — the coincidental coexistence of an adjacent naevus — must be acknowledged as a non-differential error. Nonetheless, the strengths of our study include the large sample size and the prospectively collected data in the VMS database, providing a clinically and histologically well characterised cohort of patients.
Conclusion
Consistent with earlier research, our study found that the vast majority of nodular melanomas arise in the absence of a pre-existing naevus. Melanoma development in association with a naevus was positively and independently associated with the superficial spreading melanoma subtype, location of the melanoma on the trunk, younger patient age, thinner primary tumours, and pigmented melanomas.
Most public education campaigns have focused the attention of people on observing suspicious changes in pre-existing naevi.7 While this message is undeniably important, it may be similarly imperative to emphasise the detection of suspicious de novo lesions, as melanomas of all subtypes are most likely to arise as new lesions. This is particularly the case for nodular melanoma, which contributes a disproportionately high fraction of thick primary tumours and melanoma-related deaths.1,3
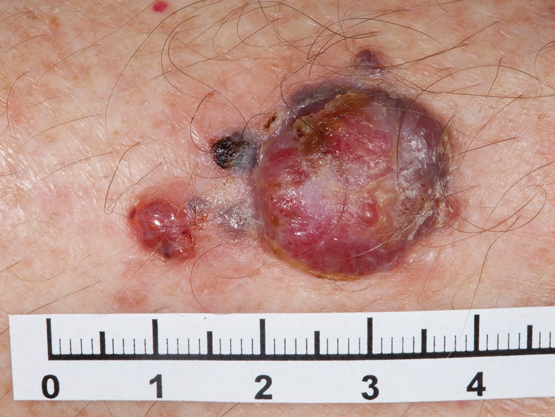
Box 1 – Nodular melanoma with five dermal and subcutaneous satellite deposits. Breslow thickness, 6.4 mm, Clark level IV (invasion into the reticular dermis), ulcerated with seven mitoses; arose de novo on the left posterior lower leg of a 66-year-old man
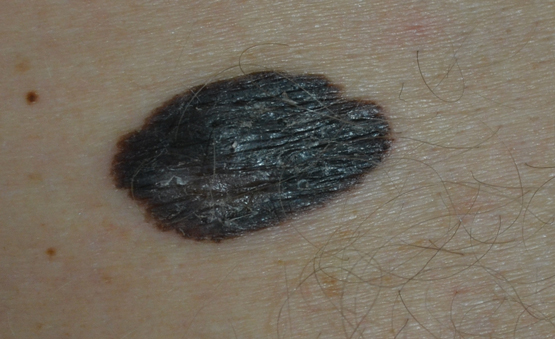
Box 2 – Superficial spreading melanoma. Breslow thickness, 1 mm, Clark level IV, non-ulcerated with one mitosis; arose within a pre-existing naevus on the left lower back of a 39-year-old man
Box 3 – Clinical and pathological characteristics of naevus-associated and de novo melanoma
|
Characteristic |
All |
Naevus-associated |
De novo |
P |
|||||||||||
|
|
|||||||||||||||
|
All melanomas |
3678 |
1360 (37.0%) |
2318 (63.0%) |
|
|||||||||||
|
Histological subtype |
|
|
|
< 0.001 |
|||||||||||
|
Superficial spreading |
3057 |
1289 (42.2%) |
1768 (57.8%) |
|
|||||||||||
|
Nodular |
621 |
71 (11%) |
550 (88.6%) |
|
|||||||||||
|
Amelanosis/hypomelanosis |
< 0.001 |
||||||||||||||
|
Yes |
283 |
45 (16%) |
238 (84.1%) |
|
|||||||||||
|
No |
3395 |
1315 (38.7%) |
2080 (61.3%) |
||||||||||||
|
Breslow thickness |
|
|
|
< 0.001 |
|||||||||||
|
≤ 1.0 mm |
1997 |
964 (48.3%) |
1033 (51.7%) |
|
|||||||||||
|
1.01–4.0 mm |
1357 |
357 (26.3%) |
1000 (73.7%) |
|
|||||||||||
|
> 4.0 mm |
279 |
36 (13%) |
243 (87.1%) |
|
|||||||||||
|
Sex |
|
|
|
0.29 |
|||||||||||
|
Men |
1943 |
735 (37.8%) |
1208 (62.2%) |
|
|||||||||||
|
Women |
1735 |
625 (36.0%) |
1110 (64.0%) |
|
|||||||||||
|
Anatomic location |
|
|
|
< 0.001 |
|||||||||||
|
Head and neck |
485 |
111 (22.9%) |
374 (77.1%) |
|
|||||||||||
|
Upper extremities |
799 |
265 (33.2%) |
534 (66.8%) |
|
|||||||||||
|
Lower extremities |
968 |
283 (29.2%) |
685 (70.8%) |
|
|||||||||||
|
Trunk |
1395 |
688 (49.3%) |
707 (50.7%) |
|
|||||||||||
|
Age group |
|
|
|
< 0.001 |
|||||||||||
|
< 30 years |
253 |
137 (54.2%) |
116 (45.8%) |
|
|||||||||||
|
30–49 years |
1213 |
545 (44.9%) |
668 (55.1%) |
|
|||||||||||
|
50–69 years |
1488 |
516 (34.7%) |
972 (65.3%) |
|
|||||||||||
|
≥ 70 years |
709 |
158 (22.3%) |
551 (77.7%) |
|
|||||||||||
|
Fitzpatrick skin type* |
0.46 |
||||||||||||||
|
I |
1020 |
362 (35.5%) |
658 (64.5%) |
|
|||||||||||
|
II |
1549 |
580 (37.4%) |
969 (62.6%) |
|
|||||||||||
|
III/IV |
1008 |
383 (38.0%) |
625 (62.0%) |
|
|||||||||||
|
Personal history of melanoma |
0.04 |
||||||||||||||
|
Yes |
518 |
210 (40.5%) |
308 (59.5%) |
|
|||||||||||
|
No |
3013 |
1077 (35.7%) |
1936 (64.3%) |
||||||||||||
|
Family history of melanoma |
0.17 |
||||||||||||||
|
Yes |
827 |
323 (39.1%) |
504 (60.9%) |
|
|||||||||||
|
No |
2843 |
1035 (36.4%) |
1808 (63.6%) |
|
|||||||||||
|
|
|||||||||||||||
|
* I = always burns, never tans; II = burns easily, tans poorly; III = burns moderately, then develops a light tan; IV = burns minimally to rarely, then develops a moderate tan; V = rarely burns, tans darkly easily; VI = never burns, always tans darkly. |
|||||||||||||||
Box 4 – Univariable and multivariable logistic regression analysis of the clinical and pathological characteristics of naevus-associated melanoma
|
Characteristic |
Univariable analysis |
Multivariable analysis* |
|||||||||||||
|
Odds ratio (95% CI) |
P |
Odds ratio (95% CI) |
P |
||||||||||||
|
|
|||||||||||||||
|
Histological subtype |
|||||||||||||||
|
Superficial spreading |
5.65 (4.37–7.31) |
< 0.001 |
3.05 (2.24–4.17) |
< 0.001 |
|||||||||||
|
Nodular |
1 |
|
1 |
|
|||||||||||
|
Amelanosis/hypomelanosis |
|||||||||||||||
|
Yes |
0.30 (0.22–0.41) |
< 0.001 |
0.68 (0.48–0.97) |
0.035 |
|||||||||||
|
No |
1 |
|
1 |
|
|||||||||||
|
Breslow thickness |
|||||||||||||||
|
Per millimetre increase |
0.62 (0.58–0.67) |
< 0.001 |
0.75 (0.69–0.81) |
< 0.001 |
|||||||||||
|
Sex |
|
|
|
|
|||||||||||
|
Men |
1.09 (0.94–1.24) |
0.30 |
1.18 (1.00–1.39) |
0.049 |
|||||||||||
|
Women |
1 |
|
1 |
|
|||||||||||
|
Age group |
|
|
|
|
|||||||||||
|
< 30 years |
1 |
|
1 |
|
|||||||||||
|
30–49 years |
0.69 (0.53–0.91) |
0.008 |
0.63 (0.46–0.86) |
0.003 |
|||||||||||
|
50–69 years |
0.45 (0.34–0.59) |
< 0.001 |
0.42 (0.31–0.57) |
< 0.001 |
|||||||||||
|
≥ 70 years |
0.24 (0.18–0.33) |
< 0.001 |
0.28 (0.20–0.40) |
< 0.001 |
|||||||||||
|
Anatomic location |
|
|
|
|
|||||||||||
|
Head and neck |
1 |
|
1 |
|
|||||||||||
|
Extremities |
1.51 (1.20–1.92) |
0.001 |
1.05 (0.80–1.37) |
0.71 |
|||||||||||
|
Trunk |
3.28 (2.59–4.15) |
< 0.001 |
2.27 (1.73–2.96) |
< 0.001 |
|||||||||||
|
Fitzpatrick skin type |
|
|
|
|
|||||||||||
|
I |
1 |
|
1 |
|
|||||||||||
|
II |
1.09 (0.92–1.28) |
0.30 |
0.98 (0.82–1.18) |
0.83 |
|||||||||||
|
III–IV |
1.11 (0.93–1.33) |
0.20 |
0.98 (0.80–1.20) |
0.85 |
|||||||||||
|
Personal history of melanoma |
|||||||||||||||
|
Yes |
1.23 (1.02–1.48) |
0.04 |
1.17 (0.94–1.45) |
0.16 |
|||||||||||
|
No |
1 |
|
1 |
|
|||||||||||
|
Family history of melanoma |
|||||||||||||||
|
Yes |
1.12 (0.95–1.31) |
0.17 |
1.05 (0.87–1.26) |
0.61 |
|||||||||||
|
No |
1 |
|
1 |
|
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Adjusted for all other covariates in this table. |
|||||||||||||||
Received 8 March 2017, accepted 13 April 2017
- Yan Pan*1,2
- Nikki R Adler*1,2
- Rory Wolfe2
- Catriona A McLean3
- John W Kelly1
- 1 Victorian Melanoma Service, Alfred Hospital, Melbourne, VIC
- 2 Monash University, Melbourne, VIC
- 3 Alfred Health, Melbourne, VIC
* Denotes equal first authors.
Nikki Adler is supported by an Australian Postgraduate Award (Australian Government Department of Industry, Innovation, Science, Research and Tertiary Education).
No relevant disclosures.
- 1. Mar V, Roberts H, Wolfe R, et al. Nodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol 2013; 68: 568-575.
- 2. Geller AC, Elwood M, Swetter SM, et al. Factors related to the presentation of thin and thick nodular melanoma from a population-based cancer registry in Queensland Australia. Cancer 2009; 115: 1318-1327.
- 3. Chamberlain AJ, Fritschi L, Giles GG, et al. Nodular type and older age as the most significant associations of thick melanoma in Victoria, Australia. Arch Dermatol 2002; 138: 609-614.
- 4. Kelly JW, Chamberlain AJ, Staples MP, et al. Nodular melanoma. No longer as simple as ABC. Aust Fam Physician 2003; 32: 706-709.
- 5. Lin MJ, Mar V, McLean C, et al. An objective measure of growth rate using partial biopsy specimens of melanomas that were initially misdiagnosed. J Am Acad Dermatol 2014; 71: 691-697.
- 6. Lin WM, Luo S, Muzikansky A, et al. Outcome of patients with de novo versus nevus-associated melanoma. J Am Acad Dermatol 2015; 72: 54-58.
- 7. Marks R, Dorevitch AP, Mason G. Do all melanomas come from “moles”? A study of the histological association between melanocytic naevi and melanoma. Australas J Dermatol 1990; 31: 77-80.
- 8. Massi D, Carli P, Franchi A, et al. Naevus-associated melanomas: cause or chance? Melanoma Res 1999; 9: 85-91.
- 9. Bevona C, Goggins W, Quinn T, et al. Cutaneous melanomas associated with nevi. Arch Dermatol 2003; 139: 1620-1624.
- 10. Sagebiel RW. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: effect of tumor thickness. J Invest Dermatol 1993; 100: 322S-325S.
- 11. Weatherhead SC, Haniffa M, Lawrence CM. Melanomas arising from naevi and de novo melanomas-does origin matter? Br J Dermatol 2007; 156: 72-76.
- 12. Purdue MP, From L, Armstrong BK, et al. Etiologic and other factors predicting nevus-associated cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev 2005; 14: 2015-2022.
- 13. Kaddu S, Smolle J, Zenahlik P, et al. Melanoma with benign melanocytic naevus components: reappraisal of clinicopathological features and prognosis. Melanoma Res 2002; 12: 271-278.
- 14. Shitara D, Nascimento MM, Puig S, et al. Nevus-associated melanomas: clinicopathologic features. Am J Clin Pathol 2014; 142: 485-491.
- 15. Gruber SB, Barnhill RL, Stenn KS, et al. Nevomelanocytic proliferations in association with cutaneous malignant melanoma: a multivariate analysis. J Am Acad Dermatol 1989; 21: 773-780.
- 16. Haenssle HA, Mograby N, Ngassa A, et al. Association of patient risk factors and frequency of nevus-associated cutaneous melanomas. JAMA Dermatol 2016; 152: 291-298.
- 17. Cymerman RM, Shao Y, Wang K, et al. De novo vs nevus-associated melanomas: differences in associations with prognostic indicators and survival. J Natl Cancer Inst 2016; 108: djw121.
- 18. Friedman RJ, Rigel DS, Kopf AW, et al. Favorable prognosis for malignant melanomas associated with acquired melanocytic nevi. Arch Dermatol 1983; 119: 455-462.
- 19. Echeverría B, Botella-Estrada R, Serra-Guillén C, et al. Increased risk of developing a second primary cutaneous nevus-associated melanoma in patients previously diagnosed with the disease. Actas Dermosifiliogr 2010; 101: 710-716.
- 20. Pizzichetta MA, Talamini R, Stanganelli I, et al. Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. Br J Dermatol 2004; 150: 1117-1124.
- 21. Thomas NE, Kricker A, Waxweiler WT, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol 2014; 150: 1306-1314.
- 22. Moreau JF, Weissfeld JL, Ferris LK. Characteristics and survival of patients with invasive amelanotic melanoma in the USA. Melanoma Res 2013; 23: 408-413.
- 23. McClain SE, Mayo KB, Shada AL, et al. Amelanotic melanomas presenting as red skin lesions: a diagnostic challenge with potentially lethal consequences. Int J Dermatol 2012; 51: 420-426.
- 24. Gualandri L, Betti R, Crosti C. Clinical features of 36 cases of amelanotic melanomas and considerations about the relationship between histologic subtypes and diagnostic delay. J Eur Acad Dermatol Venereol 2009; 23: 283-287.
- 25. Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 2011; 24: 879-897.
- 26. Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst 2003; 95: 806-812.





Abstract
Objectives: To determine the frequency of naevus-associated melanoma among superficial spreading and nodular subtypes; and to investigate associations between naevus-associated melanoma and other clinico-pathological characteristics.
Design, setting and participants: Cross-sectional study of all patients with nodular and superficial spreading melanomas diagnosed between 1994 and 2015 at the Victorian Melanoma Service, Melbourne.
Methods and main outcome measures: Clinical and pathological characteristics of naevus-associated and de novo melanomas were assessed in univariable and multivariable logistic regression analyses.
Results: Of 3678 primary melanomas, 1360 (37.0%) were histologically associated with a naevus and 2318 (63.0%) were de novo melanomas; 71 of 621 nodular (11.4%) and 1289 of 3057 superficial spreading melanomas (42.2%) were histologically associated with a naevus. In multivariable analyses, the odds of being associated with a naevus were higher for melanomas located on the trunk (v head and neck: adjusted odds ratio [OR], 2.27; 95% CI, 1.73–2.96; P < 0.001), while the odds were lower for thicker tumours (adjusted OR, 0.75 per millimetre increase in Breslow thickness; 95% CI, 0.69–0.81; P < 0.001), amelanotic/hypomelanotic melanomas (adjusted OR, 0.68; 95% CI, 0.48–0.97; P = 0.035), and older age (patients 70 years or older v patients under 30 at diagnosis: adjusted OR, 0.28; 95% CI, 0.20–0.40; P < 0.001). After adjusting for confounders, the odds of an associated naevus was three times as high for superficial spreading melanomas as for nodular melanomas (adjusted OR, 3.05; 95% CI, 2.24–4.17; P < 0.001).
Conclusion: Melanomas are most likely to arise in the absence of a pre-existing naevus, particularly nodular melanomas. Public health campaigns should therefore emphasise the detection of suspicious de novo lesions, as well as of changing lesions.