The known Keratinocyte cancer is common in Australia, but its precise incidence is not known. Earlier studies analysed aggregated Medicare data, counting the number of cancers treated; this did not account for the high proportion of patients treated for multiple lesions.
The new The incidence of keratinocyte cancer excisions was 1531 per 100 000 person-years, but varied markedly by age, sex, and region. Multiplicity is common; 74% of all skin cancers were excised from people treated for multiple lesions.
The implications Skin cancer incidence is high, but the burden is particularly high in the population subset (about half of all skin cancer patients) with multiple lesions.
Keratinocyte cancers (or non-melanoma skin cancers) are the most frequent cancers in humans,1 particularly among those of European descent. Two major types are distinguished clinically and histologically: basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs). Although the costs incurred by their diagnosis and treatment are the second highest of all cancers in Australia,2 keratinocyte cancers are often considered trivial, as most can be effectively managed in primary care. However, the morbidity of keratinocyte cancers is considerable, resulting in more than 95 000 hospital admissions (the highest for any cancer in Australia) and causing more than 500 deaths each year.3
A systematic policy response to the Australian skin cancer problem requires accurate information about patterns of incidence and morbidity. As data on keratinocyte cancers are not routinely collected by cancer registries in most jurisdictions, recent studies have drawn on summary data from administrative databases (such as Medicare) to estimate their incidence, relying on item billing codes for skin cancer treatments.4,5 While summary data have been useful for determining the scale of the problem, the lack of information on individual patients greatly limits their interpretation. For example, skin cancer multiplicity — an individual having multiple discrete skin cancer events over a given time period — could not be investigated, but all clinicians are familiar with patients with actinopathies who bear a disproportionate skin cancer burden. Similarly, the overdiagnosis of other actinic neoplasias (eg, kerato-acanthomas, intra-epidermal carcinomas) billed under skin cancer item codes cannot be assessed in administrative datasets, nor do these data distinguish between BCCs and SCCs. Finally, previous analyses have been cross-sectional and have not provided prospective estimates of skin cancer risk in Australia.
In this article, we report the findings of a detailed analysis of individual-level health administration data overlaid with histology information from a large prospective cohort study established specifically to investigate skin cancer. Our aim was to estimate the overall incidence of keratinocyte cancers, as well as incidence by histological type. We focused on identifying demographic and geographic differences in incidence, and on describing the multiplicity of skin cancer events in the Australian population.
Methods
Medicare data
Medicare is the Australian universal health insurance scheme that subsidises most medical services (but not those carried out in public hospitals, which are operated by state governments) for citizens and permanent residents. We analysed individual-level data for a systematic random sample of 10% of all persons registered with Medicare during 1997–2014, prepared by the Australian Department of Health and posted online for research purposes in August 2016.6 We restricted our analyses to people aged 20 years or more in 2011 who submitted at least one Medicare claim during 2011–2014. This period was selected for its recency, and because population-based pathology data for the same period from the QSkin study7 were available, from which we could extract age- and sex-specific BCC:SCC ratios for excised lesions. The Medicare dataset included encrypted patient identification numbers; patient sex, year of birth, and state of residence; item code number for the specific service; date of service, randomly perturbed by ±14 days; and name and state of the service provider for each claim record.
We focused on the eight Medicare Benefits Schedule (MBS) item codes reserved for keratinocyte cancer excisions (31255, 31260, 31265, 31270, 31275, 31280, 31285, 31290; online Appendix, table 1). These item numbers require that a diagnosis of BCC or SCC be confirmed by histology before being claimed by the treating doctor. The item codes do not distinguish between BCC and SCC; data from the QSkin study were analysed to estimate BCC:SCC ratios. We extracted all claims for patients with the eight codes during 2011–2014 to estimate keratinocyte cancer incidence; those with no claims under these codes were included as part of the at risk population. Individuals with one of these eight item code claims before 2011 were deemed to have a prior history of keratinocyte cancer excision.
The state of residence for the most recent claim by a patient was used for state-specific incidence estimates, grouped by Medicare for confidentiality purposes as Queensland; New South Wales and the Australian Capital Territory; Victoria and Tasmania; South Australia and the Northern Territory; and Western Australia.
In supplementary analyses, we expanded the dataset to include a further 29 item codes for destructive skin cancer therapies.4
QSkin data
We analysed data from the QSkin study7 to derive age- and sex-specific estimates of the BCC:SCC ratio. QSkin included a random sample of Queensland residents (n = 43 794) aged 40–69 years in 2010 who completed a baseline survey about skin cancer risk factors, basic demographic data, lifestyle, and medical history. Data linkage to Medicare was performed for 40 386 consenting participants, among whom we identified patients who had had keratinocyte cancer excisions coded by the eight item codes listed above. We obtained pathology reports (75% recovery) from which salient details were abstracted and recorded.
Statistical analysis
As individuals can develop multiple keratinocyte cancers over time, we estimated both person-based and lesion-based incidence rates. We used Poisson regression to estimate the crude and age- and sex-specific incidence rates for both person-based and lesion-based rates of keratinocyte cancer, BCC, and SCC. We used the Australian standard population (2001)8 to estimate age-standardised incidence rates (ASRs) of keratinocyte cancer, BCC, and SCC, in men and women. We calculated standardised incidence ratios (SIRs) for different groups from the estimated ASRs; confidence intervals for SIRs were derived from the normal approximation to log-transformed rate ratios and their standard errors.
To estimate the crude person-based incidence, we divided the total number of people with at least one excision by person-time at risk. We estimated the crude lesion-based incidence by dividing the total number of excisions of keratinocyte cancers by the total person-time at risk. We adjusted for competing mortality risks in each analysis. To estimate type-specific incidence (BCCs, SCCs), we applied our earlier finding that only 68% of excisions claimed under the eight Medicare item codes were linked to a confirmed diagnosis of BCC or SCC; the others were mostly for other actinic lesions determined by histology to be kerato-acanthomas, intra-epidermal carcinomas, Bowen disease, or related skin neoplasias.9 We therefore discounted the overall incidence of excised lesions by 32%, and then used age- and sex-specific BCC:SCC ratios from QSkin to estimate BCC and SCC incidence in our national sample.
Ethics approval
Participants in QSkin provided separate written informed consent for the collection of pathology data and for data linkage to Medicare. The QSkin Study was approved by the human research ethics committee of QIMR Berghofer Medical Research Institute (reference, P1309), and by the External Request Evaluation Committee of Medicare Australia (reference, 2010/CO06969).
Results
Study populations and risk set
The Medicare sample comprised records for 1 704 193 people aged 20 years or more in 2011 with at least one service claim during 2011–2014. Of these, 111 868 (6.6%) had at least one keratinocyte cancer excised (248 152 excisions).
According to QSkin data, the BCC:SCC ratio among first diagnosed keratinocyte cancers was higher in younger than older age groups; the ratio declined with age for both men and women (online Appendix, table 2). In all age groups, the BCC:SCC ratio was higher for women than men.
Person-based incidence of keratinocyte cancer excisions
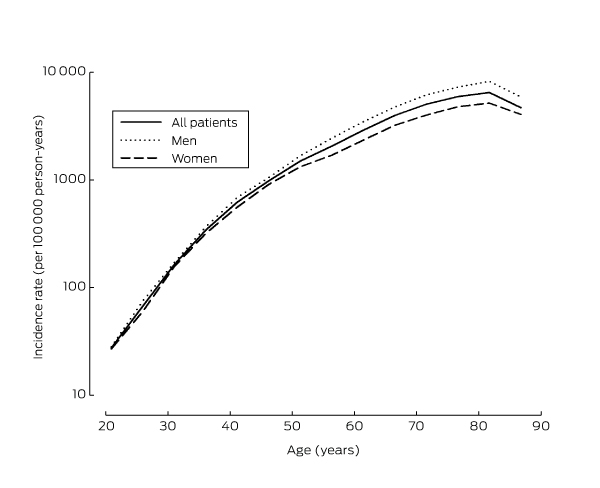
The overall rate of excisions for keratinocyte cancer in Australia was 1531 per 100 000 person-years (Box 1). The overall incidence of keratinocyte cancer treatments, including curettage, laser treatment, and liquid nitrogen cryotherapy (37 item codes), was 1796 per 100 000 person-years. The incidence increased with age, with around 80% of patients experiencing their first event at age 55 or older (Box 2). Age-specific incidence increased from 26 per 100 000 person-years among 20–24-year-old people to more than 6000 per 100 000 person-years among those aged 80–84. The incidence of keratinocyte cancer was consistently higher among men than women (SIR, 1.43; 95% CI, 1.42–1.45), and this ratio increased with age (data not shown). Incidence differed markedly between states, with rates in Queensland clearly exceeding those elsewhere. Based on the histologic subtype distribution in the QSkin data, we estimated the person-based incidence of BCC to be 770 per 100 000 person-years, and of SCC to be 271 per 100 000 person-years (Box 1).
Keratinocyte cancer multiplicity
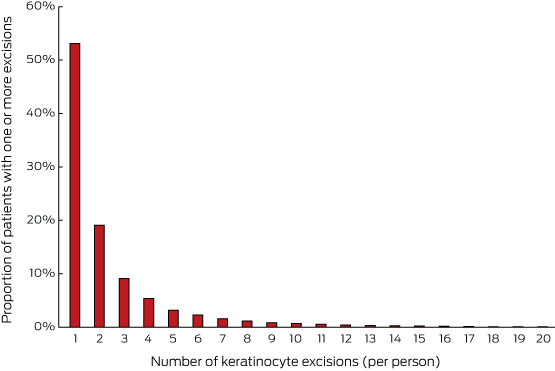
Overall, 3.9% of Australians aged 20 years or more had exactly one keratinocyte cancer excised during 2011–2014; 2.7% had had more than one excised (Box 3). Almost half of those who underwent excisions for skin cancer during the study period (47%) had two or more cancers excised (Box 4); these patients contributed 74% of all lesions excised (248 152 excisions).
A higher proportion of men than women had multiple excisions, and multiplicity was strongly correlated with age. Most male patients over 70 had been treated for multiple lesions. There were also differences in multiplicity between states, with significantly higher proportions of multiple lesions in Queensland than elsewhere (data not shown).
Rates of keratinocyte cancer by past history
People treated previously for skin cancer were much more likely to have an excision during follow-up than those without a prior history (38% v 3%). When adjusted for age and sex, the person-based rate of excisions was eight times as high for those with a history of keratinocyte cancer as for those without (SIR, 8.4; 95% CI, 8.3–8.6); the SIR varied markedly between states (online Appendix, table 4).
Lesion-based incidence
The rate of keratinocyte cancer lesion excisions in Australia during 2011–2014 was 3154 per 100 000 person-years (online Appendix, table 5); the rate of all treatments (including curettage, laser treatment, and liquid nitrogen cryotherapy) was 4458 per 100 000 person-years (data not shown). The lesion-based incidence of keratinocyte cancer was markedly higher for men than women (SIR, 1.85; 95% CI, 1.83–1.86). The SIR for Queensland was higher using lesion-based rates (SIR, 1.96; 95% CI, 1.94–1.97; online Appendix, table 5) than person-based rates (SIR, 1.75; 95% CI, 1.73–1.77; Box 1) because multiplicity was more frequent in Queensland than in other states.
Lesion-based rates of BCC and SCC in Australia were 1565 and 580 per 100 000 person-years respectively. The lesion-based incidence of SCC was about twice as high for men as for women (SIR, 2.1; 95% CI, 2.06–2.14), whereas the lesion-based incidence of BCC was about 76% higher for men than women (SIR, 1.76; 95% CI, 1.74–1.78) (online Appendix, table 5).
Discussion
In this representative sample of Australian adults aged 20 or more, about 7% had had at least one skin cancer excised during 2011–2014. Excision rates were higher for men than women and, as expected, they increased dramatically with age; about one-quarter of men aged 80 or more underwent one or more excisions during the study period. Estimates of skin cancer multiplicity in Australia have been reported only once previously, following a cross-sectional telephone survey of about 57 000 people in 2002.1 This survey recorded skin cancer events over a 12-month period, and found that 27% of people with confirmed keratinocyte cancer had more than one lesion. The survey was part of a weekly household consumer survey with a low response rate (32%), so the representativeness of the sample is difficult to determine.10 The data we have reported indicate that by the age of 70 years, about half of Australian men treated for skin cancer will have been treated for more than one lesion.
Because the Medicare sample provided longitudinal historical data, we were able to calculate incidence rates separately for those with and without a prior history of skin cancer excision; we found that rates for those with a history were more than eight times as high. This accords with our previous finding that the strongest predictor of future skin cancer risk is a past history of skin cancer.11
A notable but not unexpected finding was the geographic diversity in the incidence of keratinocyte cancers. The age-standardised rates of treatment in Queensland were nearly twice the national average and almost three times as high as in Victoria and Tasmania. When rates of treated lesions (rather than persons) were assessed, the differences between the states were further magnified, primarily because of the higher frequency of multiple lesions in Queensland.
Our estimated skin cancer incidence of 1531 per 100 000 person-years (ie, 1.5% per annum) must be compared with earlier reports for Australia. On the basis of summary Medicare data, Fransen and colleagues4 calculated an age-standardised treatment rate for keratinocyte cancers (2011) of 3271 per 100 000 person-years. However, the authors analysed numbers of services provided — that is, lesion-based analyses — and therefore did not adjust for multiple, separate skin events in individual patients. As we found by analysing individual-level data, skin cancer multiplicity is common. Secondly, Fransen and colleagues4 included MBS item codes for destructive therapies as well as for excisions. When we used the same item codes, we estimated an ASR of 4458 cases per 100 000 person-years, 36% higher than the 2011 estimate, probably attributable to differences in the at risk populations of the two studies (the earlier study included the entire Australian population). We intentionally restricted our primary analysis to item codes for excisions, as they are by far the most frequently claimed items for treating skin cancer. Moreover, we have previously reported that item codes for excisions accord reasonably well with histology report results,9 whereas the extent of concordance with other MBS items is unknown.
Strengths of our analysis included the extremely large size and representativeness of the sample, the ability to analyse individual-level data over an extended period, and the inclusion of histology-specific data from a large population cohort being tracked for skin cancer outcomes. These features permitted more detailed analyses than in earlier reports, and accounted for lesion multiplicity and prior history of treatments for skin cancer. Importantly, we were able to estimate the burdens of BCC and SCC separately, and to adjust our estimates for anticipated overdiagnosis.
The limitations of our analyses include the fact that the Medicare sample includes only eligible people who had made at least one claim for a service; bias might arise if the age and sex distributions of people in the Medicare sample differed from those of the overall Australian population. There was a slightly lower proportion of men under 30 in the Medicare sample than in the average estimated resident population of Australia for the study period. In supplementary analyses (data not shown), increasing the denominator by 10% for men in this age group did not alter the estimated ASR; it is therefore unlikely that non-representativeness of the Medicare sample seriously biased our estimates of skin cancer incidence. The health care of about 190 000 Australians (fewer than 1% of the population) is funded by the Department of Veterans Affairs (DVA) and therefore not captured in Medicare data. However, the age-standardised rates of skin cancer in the DVA population have not been reported to be higher than for the general population, and their exclusion from our analyses is unlikely to have markedly influenced our estimates.
Excluding lesions treated destructively probably also means our incidence estimates are conservative, as a proportion of the lesions treated destructively will be keratinocyte cancers. We have no data on how many newly incident skin cancers arise each year but are not excised, further depressing our estimates of keratinocyte cancer incidence. We did not count prescriptions for topical treatments of actinic lesions (such as imiquimod, diclofenac, ingenol mebutate), as they are predominantly employed to treat non-malignant lesions. Moreover, as Medicare data indicate that excisions of histologically confirmed skin cancers are about 30 times as frequent as prescriptions for topical treatments, any underestimation of incidence caused by omitting topical creams from the analysis is negligible.
To estimate the population incidence rates of BCC and SCC, we used the age- and sex-specific ratio of these histologic types from the QSkin study, a large, prospective study for which we had access to pathology records. However, it is possible that the ratio for the QSkin population (Queensland residents) may not be generalisable to the rest of Australia. While internationally BCC:SCC ratios vary with latitude,12,13 the limited data available for Australia suggest that the ratio is similar across geographic regions (northern, central, southern Australia).1 Further, our estimate that 32% of claims for keratinocyte cancer excisions were for lesions other than BCCs or SCCs may apply only to Queensland; overdiagnosis may be higher there than elsewhere because multiplicity rates are higher, and Queensland patients are therefore more likely to undergo excisions of suspicious lesions than other Australian residents. We would then have underestimated the incidence of BCCs and SCCs in states other than Queensland.
In summary, our analyses document the person-based incidence of keratinocyte cancers for the Australian population, and provide the most comprehensive assessment of their occurrence by age, sex, state, and prior history of skin cancer. The reported incidence rates are extremely high when compared with overseas rates, and underscore the magnitude of the burden of skin cancer in Australia.
Box 1 – Absolute and relative incidence of keratinocyte cancers (person-based), and estimated incidence of basal (BCC) and squamous cell carcinomas (SCC), Australia, 2011–2014
|
|
Keratinocyte cancer incidence (per 100 000 person-years)* |
Standardised incidence ratio (95% CI) |
BCC incidence (per 100 000 person-years) |
SCC incidence (per 100 000 person-years) |
|||||||||||
|
Women |
Men |
All |
Women |
Men |
All |
Women |
Men |
All |
|||||||
|
|
|||||||||||||||
|
Australia |
1272 |
1824 |
1531 |
1.00 |
656 |
899 |
770 |
209 |
341 |
271 |
|||||
|
Queensland |
2296 |
3105 |
2679 |
1.75 (1.73–1.77) |
1191 |
1538 |
1355 |
371 |
573 |
467 |
|||||
|
New South Wales/Australian Capital Territory |
1217 |
1809 |
1495 |
0.98 (0.96–0.99) |
626 |
891 |
751 |
201 |
339 |
266 |
|||||
|
Victoria/Tasmania |
800 |
1158 |
966 |
0.63 (0.62–0.64) |
410 |
567 |
482 |
135 |
221 |
175 |
|||||
|
Western Australia |
1216 |
1744 |
1467 |
0.96 (0.94–0.98) |
625 |
859 |
736 |
201 |
328 |
261 |
|||||
|
South Australia/Northern Territory |
909 |
1386 |
1132 |
0.74 (0.72–0.76) |
466 |
679 |
565 |
153 |
264 |
205 |
|||||
|
|
|||||||||||||||
|
CI = confidence interval. * Incidence age standardised to the Australian standard population (2011). |
|||||||||||||||
Box 2 – Age-specific incidence of keratinocyte cancers (person-based) in people aged 20 years or more, Australia, 2011–2014
Box 3 – Number of keratinocyte cancers excised per person in Australia, 2011–2014, by age and sex*
|
Age group |
All people |
Women |
Men |
||||||||||||
|
None |
One |
Two or more |
None |
One |
Two or more |
None |
One |
Two or more |
|||||||
|
|
|||||||||||||||
|
20–39 years |
99.2% |
0.6% |
0.2% |
99.3% |
0.5% |
0.1% |
99.2% |
0.6% |
0.2% |
||||||
|
40–59 years |
94.7% |
3.6% |
1.7% |
95.4% |
3.3% |
1.3% |
94.0% |
3.9% |
2.2% |
||||||
|
60–79 years |
84.0% |
8.8% |
7.2% |
86.8% |
8.0% |
5.2% |
81.0% |
10.0% |
9.4% |
||||||
|
≥ 80 years |
80.2% |
9.9% |
9.9% |
83.6% |
9.2% |
7.2% |
74.9% |
11.1% |
14.0% |
||||||
|
Overall |
93.4% |
3.9% |
2.7% |
94.4% |
3.6% |
2.0% |
92.5% |
4.1% |
3.4% |
||||||
|
|
|||||||||||||||
|
* Absolute numbers for this table are included in the online Appendix as table 3. |
|||||||||||||||
Received 24 March 2017, accepted 30 June 2017
- Nirmala Pandeya
- Catherine M Olsen
- David C Whiteman
- QIMR Berghofer Medical Research Institute, Brisbane, QLD
We are grateful to Jonathan Davies and Archie De Guzman (QIMR Berghofer IT Department) for their help in obtaining and managing Medicare data and extracting the relevant subset for this analysis. This work is supported by the National Health and Medical Research Council (grant numbers 1073898, 1058522). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No relevant disclosures.
- 1. Staples MP, Elwood M, Burton RC, et al. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust 2006; 184: 6-10. <MJA full text>
- 2. Australian Institute for Health and Welfare. Health system expenditure on cancer and other neoplasms in Australia, 2008–09 (AIHW Cat. No. CAN 78; Cancer Series No. 8). Canberra: AIHW, 2013.
- 3. Australian Institute for Health and Welfare, Australasian Association of Cancer Registries. Cancer in Australia: an overview 2012 (AIHW Cat. No. CAN 70; Cancer Series No. 74). Canberra: AIHW, 2012.
- 4. Fransen M, Karahallos A, Sharma N, et al. Non-melanoma skin cancer in Australia. Med J Aust 2012; 197: 565-568. <MJA full text>
- 5. Olsen CM, Williams PF, Whiteman DC. Turning the tide? Changes in treatment rates for keratinocyte cancers in Australia 2000 through 2011. J Am Acad Dermatol 2014; 71: 21-26.e1.
- 6. Medicare Australia. Linkable de-identified 10% sample of Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Schedule (PBS) Australia. 2016. https://researchdata.ands.org.au/linkable-de-identified-schedule-pbs/673945 (accessed July 2017).
- 7. Olsen CM, Green AC, Neale RE, et al. Cohort profile: the QSkin Sun and Health Study. Int J Epidemiol 2012; 41: 929-929i.
- 8. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, Mar 2013. Sept 2013. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Mar%202013?OpenDocument (accessed Jan 2017).
- 9. Thompson BS, Olsen CM, Subramaniam P, et al. Medicare claims data reliably identify treatments for basal cell carcinoma and squamous cell carcinoma: a prospective cohort study. Aust N Z J Public Health 2016; 40: 154-158.
- 10. National Cancer Control Initiative. The 2002 national non-melanoma skin cancer survey. A report by the NCCI Non-melanoma Skin Cancer Working Group. Melbourne: NCCI, 2003. https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/2002-national-non-melanoma-skin-cancer-survey (accessed July 2017).
- 11. Whiteman DC, Thompson BS, Thrift AP, et al. A model to predict the risk of keratinocyte carcinomas. J Invest Dermatol 2016; 136: 1247-1254.
- 12. Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol 2015; 151: 1081-1086.
- 13. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069-1080.





Abstract
Objectives: To assess the incidence and multiplicity of keratinocyte cancers (basal cell carcinoma [BCC] and squamous cell carcinoma [SCC]) excised in Australia, and to examine variations by age, sex, state, and prior skin cancer history.
Design: Analysis of individual-level Medicare data for keratinocyte cancer treatments (identified by eight specific MBS item codes) during 2011–2014. Histological data from the QSkin prospective cohort study were analysed to estimate BCC and SCC incidence.
Setting: A 10% systematic random sample of all people registered with Medicare during 1997–2014.
Participants: People aged at least 20 years in 2011 who made at least one claim for any MBS medical service during 2011–2014 (1 704 193 individuals).
Main outcome measures: Age-standardised incidence rates (ASRs) and standardised incidence ratios (SIRs).
Results: The person-based incidence of keratinocyte cancer excisions in Australia was 1531 per 100 000 person-years; incidence increased with age, and was higher for men than women (SIR, 1.43; 95% CI, 1.42–1.45). Lesion-based incidence was 3154 per 100 000 person-years. The estimated ASRs for BCC and SCC were 770 per 100 000 and 270 per 100 000 person-years respectively. During 2011–2014, 3.9% of Australians had one keratinocyte cancer excised, 2.7% had more than one excised; 74% of skin cancers were excised from patients who had two or more lesions removed. Multiplicity was strongly correlated with age; most male patients over 70 were treated for multiple lesions. Keratinocyte cancer incidence was eight times as high among people with a prior history of excisions as among those without.
Conclusions: The incidence and multiplicity of keratinocyte cancer in Australia are very high, causing a large disease burden that has not previously been quantified.