Summary
- There is no convincing evidence that classic Lyme disease occurs in Australia, nor is there evidence that the causative agent, Borrelia burgdorferi, is found in Australian animals or ticks.
- Lyme disease, however, can be acquired overseas but diagnosed in Australia; most people presenting with laboratory-confirmed Lyme disease in Australia were infected in Europe.
- Despite the lack of evidence that Lyme disease can be acquired in Australia, growing numbers of patients, their supporters, and some politicians demand diagnoses and treatment according to the protocols of the “chronic Lyme disease” school of thought.
- Antibiotic therapy for chronic “Lyme disease-like illness” can cause harm to both the individual (eg, cannula-related intravenous sepsis) and the broader community (increased antimicrobial resistance rates).
- Until there is strong evidence from well performed clinical studies that bacteria present in Australia cause a chronic debilitating illness that responds to prolonged antibiotics, treating patients with “Lyme disease-like illness” with prolonged antibiotic therapy is unjustified, and is likely to do much more harm than good.
Despite a number of reports of putative cases and a discussion across several decades,1-19 locally acquired classic Lyme disease has not been identified in Australia. Despite intensive efforts, the bacteria that cause Lyme disease, Borrelia species collectively termed the Borrelia burgdorferi sensu lato (B. burgdorferi s.l.) complex, have not been cultured from any definite locally acquired cases of the disease. Further, Australia does not appear to have a competent tick vector for these species.8,13,18 Finally, bacterial DNA has not been definitively detected in patients for whom acquisition in a country where B. burgdorferi is known to be endemic could be excluded.10,18
The controversy is not restricted to whether B. burgdorferi s.l. and a competent tick vector exist in Australia. We also need to consider whether chronic Lyme disease exists here. This concept does not require the aetiological agent to be metabolically active beyond maintaining a resting metabolism; it need only be present in the patient and viable. Further, the term “chronic Lyme disease” is not consistently defined: it has been applied to patients who present with active, previously untreated B. burgdorferi s.l. infections, to those who have persistent symptoms after being treated for Lyme borreliosis, to people who have had Lyme borreliosis in the past but whose current illness is unrelated to that infection, and to patients without any history of borreliosis. In Australia, substantial numbers of patients without evidence of current or past B. burgdorferi s.l. infection have been labelled with “chronic Lyme” or “Lyme-like disease”, often after bites by Australian ticks. However, even in countries where classic Lyme disease is endemic, the mainstream medical position is that persistence of infection has not been demonstrated in vivo; lingering, non-specific symptoms appear to be post-infectious sequelae unrelated to ongoing active infection.
Internationally, the concept of chronic Lyme disease polarises opinion. In the United States, the key protagonists in the debate are the Infectious Diseases Society of America (IDSA), an association of physicians and medical scientists, and the public advocacy group, the International Lyme and Associated Diseases Society (ILADS).20,21 Consistent with its model of persistent infection, ILADS and practitioners who share its views15,17,20,22 advocate long term treatment with oral antibiotics and sometimes prolonged use of intravenous antibacterial agents and associated complementary therapies, such as probiotics and natural and alternative therapies, for managing the adverse effects of long term antimicrobial administration.
Laboratory diagnosis of Lyme disease remains a significant challenge
Some people believe they have acquired Lyme disease in Australia because the results of screening antibody tests to B. burgdorferi are positive. However, instances in which there was no overseas exposure are all likely to be false positive test results. All diagnostic tests produce both false positive and false negative results; their frequency depends on the specificity and sensitivity of the test and the prevalence of the disease in the population tested. Even a highly specific test will produce some false positives, so that people who have never been exposed to B. burgdorferi can have reactive antibody results.
Diagnosis is further complicated by antigenic variations in the organisms used for developing these assays. Not only are there antigenic differences between B. burgdorferi s.l. species, but many of their genes are differentially expressed in tick and mammal environments. The sensitivity of detection is low early in the infection (less than 50% during the first week), but increases with time. Specific antibodies are usually detectable in people presenting with late manifestations of disease, such as arthritis.21
To improve specificity, a two-tiered serology testing algorithm has been internationally recommended,23,24 including western blot testing with strict interpretative criteria. Second assays are similarly used to maximise specificity when diagnosing human immunodeficiency virus and syphilis infections. Recombinant purified antigens and peptides derived from the bacterial surface lipoprotein VlsE (C6 peptide) have improved the sensitivity of detection of B. burgdorferi tests while maintaining specificity both in screening assays and immunoblots.
The best independent confirmation of any reactive antibody result is demonstrating the microorganism itself. This usually involves culturing the microbe or detecting its genome by polymerase chain reaction (PCR). PCR targeting various gene targets (flaB, 16SrRNA, recA, p66, ospA, 5SrRNA–23SrRNA gene spacer region) can provide highly specific evidence of B. burgdorferi nucleic acid, but the very low organism load means that even the sensitivity of PCR in this context is not great.25 Further, if too many PCR cycles are undertaken, specificity is lost; there is also the possibility of contamination. Culturing the Borrelia spirochaete is difficult. The number of spirochaetes in clinical specimens (skin, blood, cerebrospinal fluid, synovial fluid) is low, and their culture requires special media and prolonged incubation. As a result, antibody tests, despite their problems, remain the primary basis of diagnosis.
If a patient both receives a confirmed reactive antibody result from an appropriately accredited medical testing laboratory and presents with symptoms consistent with the relevant disease, the predictive value of the positive test result increases. However, these conditions do not appear to have been met for any patient diagnosed with possible Lyme disease acquired in Australia. Further, we need to resist requests by patients and others to provide assays that lack clinical validation, including testing urine for B. burgdorferi antigens, lymphocyte transformation tests, and in-house antibody assays that often apply interpretative criteria different to those of commercially approved assays.26
What are the results of antibody testing in Australia?
A large private diagnostic laboratory that conducts about 250 serological tests for Lyme disease each month provided the samples discussed in this article. Referrals came from all Australian states, with most from New South Wales (45% of all tests) and Queensland (27%); women aged 30–50 years were the largest group tested (Box 1, Box 2, Box 3). Over a 23-month period (September 2014 – July 2016), 5372 of 5628 tests (95.5%) in 5395 patients returned negative results (Box 4). Two-tier testing, including an immunoassay followed by an immunoblot, was performed on the 256 samples (4.5%) for which the screening results were equivocal or positive; the western blot results for three-quarters of these samples (177 of 256) were negative, and the screening results were classified as false positive results. Seventy-nine samples (1% of all samples) returned positive results for both the screen immunoassay and an initial immunoblot. Results for a large subset of these patients (29 of 79, or a further 0.5% of all tests) with a low pre-test probability of infection (no relevant symptoms or epidemiological risk factors) were negative on a second immunoblot; the total number of true positive tests was therefore 50 (0.9% of all tests), from a total of 43 patients. The total number of false positive results was 206 of 256 positive screening tests, or 80.5%. These results highlight the fact that commercially available systems use different recombinant antigens and apply different criteria for a positive result. Because of these variations, this second tier of testing should be regarded as an additional test, with a greater emphasis on specificity, supporting the clinical diagnosis rather than confirming it.27
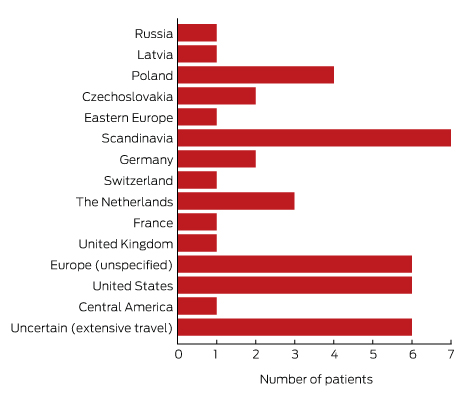
To minimise the risk of a false positive result, tests should be requested only if there is a well founded clinical suspicion of Lyme disease, and not in situations of low pre-test probability.23 A travel history was available for 37 of the 43 patients with true positive results; all had returned from countries in which Lyme disease is endemic. Most Lyme disease acquired overseas but diagnosed in Australia was European in origin (30 of 43, or 70% of cases; Box 5).
Background to the belief that Lyme disease exists in Australia
Since the early 1990s, the Australian medical community, especially specialist microbiologists and infectious diseases physicians, have debated whether an indigenous form of classic Lyme diseases occurs in Australia, especially in areas with high rates of tick bites.2-4,8-12,15,17,18 This interest motivated some of the early tick surveys.2,17 In 1991, B. burgdorferi s.l. could not be confirmed in any of 176 tick species examined.2,17 The findings of more recent surveys have also been negative.16,19
Native and introduced animals have also been investigated. The presence of Borrelia spp. in native fauna was reported by Mackerras1 and Carley and Pope2 during the 1950s and 1960s. A human volunteer was inoculated with B. queenslandica, but without causing disease,2 and it is very unlikely that B. queenslandica can induce an illness like classic Lyme disease.
The most frequently cited Borrelia species identified in introduced fauna are B. theileri (bovine borreliosis) and B. anserina (avian spirochaetosis).28 Neither of these bacteria causes a disease in humans consistent with classic Lyme disease or a chronic debilitating illness that manifests as a constellation of chronic non-specific symptoms. Neither species is part of the B. burgdorferi s.l. complex as currently defined.
Some medical practitioners in Australia became aware of Lyme disease soon after this emerging disease was described in the northeastern United States. Travellers from endemic areas were diagnosed with Lyme disease when serological tests became available. As the controversy in the US about chronic Lyme disease intensified, patients in Australia began presenting with non-specific symptoms that they related to the putative disorder, such as chronic fatigue, cognitive impairment, myalgias, and arthralgias. These patients were often clustered around a small number of general practitioners who, assessing their symptoms as being consistent with chronic Lyme disease, requested laboratory testing. Most tests undertaken in Australian laboratories returned negative results; specimens were then frequently sent to overseas laboratories, often to facilities describing themselves as being specialised for Lyme and associated diseases. Some of these laboratories reported positive results, interpreted by the treating medical practitioner as confirming their clinical diagnosis of chronic Lyme disease.
As the volume of information shared by patients and their supporters, both medical and non-medical, increased in Australia, their numbers also grew significantly. Online sharing of information via social media and digital forums has brought together thousands of Australians who believe they have Lyme disease. There are now patient advocacy groups in most states and territories, the two most prominent being the Lyme Disease Association of Australia and the Karl McManus Foundation.
Given the lack of evidence that Australia has either the aetiological agent or competent vector required for classic Lyme disease, many advocates have adopted the new label, “Lyme disease-like illness”.29 The problem with this term is that it suggests that chronic Lyme disease is a recognised medical diagnosis, whereas its validity remains contentious. Another description used is “multi-systemic infectious diseases syndrome” (MSIDS), despite the fact that it has not been established that the illness denoted by this term is infectious, nor that its constellation of non-specific symptoms is post-infectious. Environmental toxins and psychological bases have not been excluded as explanations. Moreover, many patients are initially diagnosed with neurological disorders, including motor neurone disease, Parkinson disease, multiple sclerosis and Alzheimer disease, and some advocates claim that these chronic neurological conditions are also caused by Lyme borreliosis.30,31
Clinicians and medical scientists in Australia are able to identify and fully characterise unexpected or previously unknown pathogens, as evidenced by the recent report of a Babesia infection.32 Western Australian researchers recently described a novel Borrelia species in Australian ticks,16,19,33 and many in the “Lyme disease” community are interested in this novel bacterium and the possibility that it might cause their illness. It has, however, not yet been shown to be pathogenic. Many patients and their medical practitioners believe that further pathogens play a significant role in “Lyme disease”, and that co-infection by Babesia, Anaplasma, Bartonella and Ehrlichia species are commonplace.17,20 Although these pathogens are carried by ticks, tick-borne infections caused by these microorganisms are not often diagnosed in Australia. To further confound matters, some chronic Lyme disease advocates argue that ticks are not the only vector for infection, with mosquitoes, midges, sand flies, and even leeches cited as potential alternatives.
While there is no compelling evidence that classic Lyme disease exists in Australia, Australians do acquire unusual novel infections32,33 as well as more commonly recognised infections from ticks (eg, rickettsiosis). Some may have illnesses caused by tick-borne bacteria or viruses that are yet to be identified but which may be widely distributed in Australia.
The Senate inquiry
A recent Senate inquiry raised questions about the epidemiology, diagnosis, treatment, and investigation of Lyme disease. The inquiry began on 12 November 2015, and, because of the double dissolution election on 2 July 2016, the committee issued an interim report on 4 May 2016.29 The inquiry was re-adopted by the 45th Parliament of Australia on 13 September 2016, with a reporting date of 30 November 2016.
The stigma felt by patients with a chronic debilitating illness was a major focus of the Senate inquiry. We believe that the most effective way to reduce this stigma is to concentrate appropriately funded research and clinical studies on discovering any bacterial or viral causes for such a chronic debilitating illness. This is best achieved with a One Health approach incorporating disease management elements of human, animal and environmental health care, investigating not only human infections, but also animal reservoirs and vectors that might harbour microorganisms which cause illness in people or animals.
The reported chronic debilitating symptoms overlap to a considerable degree with those of chronic fatigue syndrome (CFS) and related disorders. In most patients diagnosed with CFS, the exact cause is unknown, although Epstein-Barr virus, cytomegalovirus, and other viruses have all been implicated in syndromes characterised by fatigue-like symptoms that can persist for more than 6 months.
If we focus attention on patients with constellations of chronic debilitating symptoms in Australia, diagnostic assessment will initially include a research component (eg, metagenomic analysis, employing next generation sequencing). This will be of great benefit for identifying the many uncharacterised viruses and bacteria that probably occur in ticks, animals and people in Australia, and should inform diagnostic methods that can be included in the scope of accreditation for medical testing laboratories.
What are the problems for patients, the Australian community and doctors?
The dangers of intravenous therapy
It is vital that medical practitioners not do more harm than good with any intervention they undertake. While the questions of whether persistent B. burgdorferi infection occurs and classic Lyme disease exists in Australia can be debated, there is no denying that receiving intravascular antibiotics is associated with the risk of bloodstream infections by bacteria and fungi, in many cases fatal,34-36 including in some people treated for “Lyme disease”.36 The mortality rate associated with Staphylococcus aureus bloodstream infections is 15–35%.35 The major medical cost of prolonged intravenous therapy is thus accompanied by social costs to the patient, who should therefore not receive prolonged intravenous therapy if there is no clear evidence that it will be beneficial.
Antibiotic overuse and misuse
Many people who believe they have Lyme disease or Lyme disease-like illness, as well as some of their medical practitioners, also believe that prolonged antibiotic therapy, including intravenous antibiotics, may cure their disease.20 Evidence from the US and Europe, where classic Lyme disease is endemic, do not confirm this view. In particular, prolonged intravenous antibiotic therapy (longer than one month)37-40 does not seem to significantly improve symptoms.
Antibiotic resistance resulting from the unnecessary and prolonged use of broad spectrum antibiotics (eg, ceftriaxone) is a major problem. Antibiotic resistance not only harms the person receiving the agent (who will often be colonised by more resistant bacteria) but also the broader community: when resistant bacteria develop or multiply in an individual, they can be spread to family members and to the wider public.
Advocates of long term antimicrobial therapy point to acne, tuberculosis and Hansen disease as examples of diseases in which long term antibiotic therapy has not raised the ire of experts concerned with antimicrobial resistance. This, however, is not true, especially with respect to acne.41 Further, advocates of long term antibiotic therapy for “Lyme disease” do not appreciate that generalisations cannot be made when treating infections caused by different genera and species of bacteria.
Other potential hazards of taking antibiotics unnecessarily include their toxicity, potential hypersensitivity reactions and even anaphylaxis (allergy), and predisposition to infection with Clostridium difficile and antibiotic-resistant bacteria.42
Dilemmas for Australian doctors
Australian medical practitioners are faced with a difficult dilemma. Growing numbers of patients, their supporters, some integrative medical practitioners, and politicians are demanding diagnoses and treatment according to the protocols of the “chronic Lyme disease” school of thought. This situation is reminiscent of campaigns by advocacy groups, who mounted pressure on legislators and policy makers in the US and Canada, leading to the introduction of parliamentary bills.43,44 Both chambers of our parliament29,45 have issued reports that include recommendations for the investigation of and research into “chronic Lyme disease”, as well as for developing a case definition of this debilitating illness.
However, evidence of a local aetiological agent and a competent vector that together could cause classic Lyme disease in Australia has yet to be reported. Novel viruses and bacteria have recently been discovered in Australian ticks.16,19,25,32,46 Further research, using next generation sequencing and metagenomics, may identify links between specific tick-borne pathogens and patients who present with this constellation of chronic, non-specific symptoms, and it is only after such a link has been established that effective, evidence-based management protocols can be safely developed.
Conclusion
With the advent of new molecular scientific tools, it would not be astonishing were new viral or bacterial pathogens discovered, in ticks and other vectors, that cause acute or chronic infections or symptoms in humans in Australia. However, there is no convincing evidence that classic Lyme disease occurs here; in particular, there is no evidence that a species from the B. burgdorferi s.l. complex occurs in Australian animals or ticks. Until there is strong evidence from well performed clinical studies that bacteria present in Australia cause a chronic debilitating illness that responds to extended antibiotic therapy, treating patients with “Lyme disease-like illness” with prolonged intravenous or oral antibiotic therapy is both unjustifiable and unethical, and is likely to do much more harm than good. This harm affects both the individual (eg, intravenous sepsis) and the Australian community (increased antimicrobial resistance rates).
While immediate treatment solutions for patients presenting with these symptoms are not readily available, we strongly recommend a multidisciplinary approach to the investigation, diagnosis and management of “chronic Lyme disease”. This is especially important for ensuring that alternative diagnoses are not missed or ignored. Engaging with general practitioners, specialist physicians and microbiologists is critical for helping each patient.
Box 1 – Lyme antibody test requests by month and year, Australia, September 2014 – July 2016*
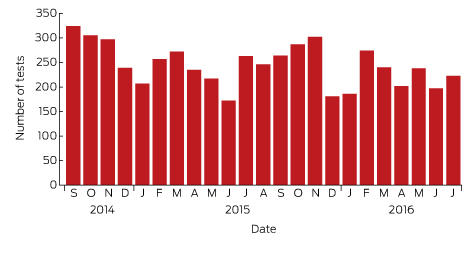
Source: Sonic Pathology (Sullivan Nicolaides Pathology, Brisbane). * Total number of requests: 5628; total number of patients: 5395.
Box 2 – Origin of 5628 Lyme antibody test requests, Australia, September 2014 – July 2016, by state and territory
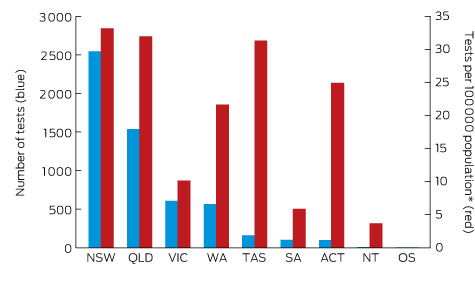
OS = overseas. * Based on 2015 Australian Bureau of Statistics population data.
Box 3 – Lyme antibody test requests, Australia, September 2014 – July 2016, by age group and sex of patients*
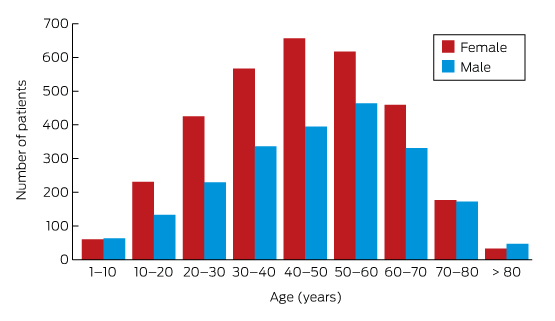
* Total number of requests: 5628; total number of patients: 5395.
Box 4 – The results of Lyme disease antibody test requests, Australia, September 2014 – July 2016
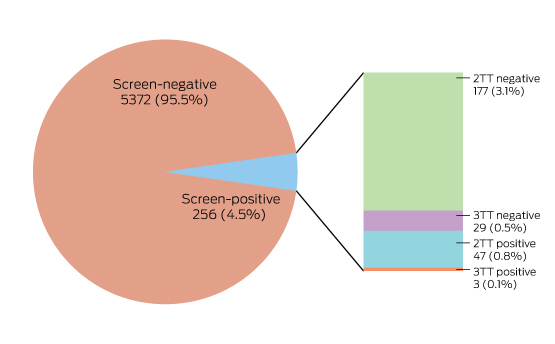
In two-tier testing (2TT), the screening assay used was the recombinant VlsE-based Liaison chemiluminescence immunoassay for Borrelia burgdorferi (IgG) (DiaSorin); the second tier test employed was the anti-Borrelia burgdorferi IgG Euroline-RN-AT immunoblot system (Euroimmun). For the second series of immunoblot tests (3TT; performed on a subset of mostly low pre-test probability specimens), an in-house IgG western immunoblot for B. burgdorferi and B. afzelii, or the MarDx IgG EU Lyme (B. afzelii, B. garinii) + VlsE western blot system (Trinity Biotech) was used. 3TT was performed at Pathology West – ICPMR Westmead, Sydney.
Provenance: Not commissioned; peer reviewed.
- Peter J Collignon1,2
- Gary D Lum2,3
- Jennifer MB Robson4
- 1 Australian National University, Canberra, ACT
- 2 ACT Pathology, Canberra Hospital, Canberra, ACT
- 3 Australian Government, Health Department, Canberra, ACT
- 4 Sullivan Nicolaides Pathology, Brisbane, QLD
No relevant disclosures.
- 1. Mackerras MJ. The haematozoa of Australian mammals. Aust J Zool 1959; 7: 105-135.
- 2. Carley JG, Pope JH. A new species of Borrelia (B. queenslandica) from Rattus villosissimus in Queensland. Aust J Exp Biol Med 1962; 40: 255-262.
- 3. Lawrence RH, Bradbury R, Cullen JS. Lyme disease on the NSW central coast. Med J Aust 1986; 145: 364.
- 4. McCrossin I. Lyme disease on the NSW south coast. Med J Aust 1986; 144: 724-725.
- 5. Wills MC, Barry RD. Detecting the cause of Lyme disease in Australia. Med J Aust 1991; 155: 275.
- 6. Baldock FC, Yamane I, Gardner I. Pilot survey for Lyme disease antibodies in Brisbane dogs. Aust Vet J 1993; 70: 356-357.
- 7. Russell RC, Doggett SL, Munro R, et al. Lyme disease: a search for a causative agent in ticks in south-eastern Australia. Epidemiol Infect 1994; 112: 375-384.
- 8. Russell RC. Lyme disease in Australia — still to be proven! Emerg Infect Dis 1995; 1: 29-31.
- 9. Playford G, Whitby M. Tick-borne diseases in Australia. Aust Fam Physician 1996; 25: 1841-1845.
- 10. Hudson BJ, Stewart M, Lennox VA, et al. Culture-positive Lyme borreliosis. Med J Aust 1998; 168: 500-502.
- 11. Nash PT. Does Lyme disease exist in Australia? Med J Aust 1998; 168: 479-480.
- 12. Russell RC. Vectors vs. humans in Australia — who is on top down under? An update on vector-borne disease and research on vectors in Australia. J Vector Ecol 1998; 23: 1-46.
- 13. Piesman J, Stone B. Vector competence of the Australian paralysis tick, Ixodes holocyclus, for the Lyme disease spirochete Borrelia burgdorferi. Int J Parasitol 1999; 21: 109-111.
- 14. Brakoulias V. Lyme disease or a complication of delusional parasitosis? Aust N Z J Psychiatry 2014; 48: 97-98.
- 15. Mayne P, Song S, Shao R, et al. Evidence for Ixodes holocyclus (Acarina: Ixodidae) as a vector for human Lyme borreliosis infection in Australia. J Insect Sci 2014; 14: 1-3.
- 16. Gofton AW, Doggett S, Ratchford A, et al. Bacterial profiling reveals novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma species in Australian human-biting ticks. PLoS One 2015; 10: e0145449.
- 17. Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int J Gen Med 2015; 8: 15-26.
- 18. Chalada MJ, Stenos J, Bradbury RS. Is there a Lyme-like disease in Australia? Summary of the findings to date. One Health 2016; 2: 42-54.
- 19. Loh SM, Gofton AW, Lo N, et al. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasit Vectors 2016; 9: 339.
- 20. Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther 2014; 12: 1103-1135.
- 21. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43: 1089-1134.
- 22. McFadzean N. Lyme disease in Australia, fundamentals of an emerging epidemic. South Lake Tahoe, Calif: BioMed Publishing Group, 2012.
- 23. Moore A, Nelson C, Molins C, et al. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis 2016; 22: 1169-1177.
- 24. Royal College of Pathologists of Australasia. Diagnostic laboratory testing for borreliosis (“Lyme disease” or similar syndromes) in Australia and New Zealand [position statement]. Mar 2016. Sydney: RCPA, 2014. http://www.rcpa.edu.au/Library/College-Policies/Position-Statements/Diagnostic-Laboratory-testing-for-Borreliosis-Lyme (accessed July 2016).
- 25. Aguero-Rosenfeld ME, Wang G, Schwartz I, et al. Diagnosis of Lyme borreliosis. Clin Microbiol Rev 2005; 18: 484-509.
- 26. Centers for Disease Control and Prevention. Notice to readers: caution regarding testing for Lyme disease. MMWR Morb Mortal Wkly Rep 2005; 54: 125.
- 27. Robertson J, Guy E, Andrews N, et al. A European multicenter study of immunoblotting in serodiagnosis of Lyme borreliosis. J Clin Microbiol 2000; 38: 2097-2102.
- 28. Seddon HR. Diseases of domestic animals in Australia. Part 5. Bacterial disease (Service publications [veterinary hygiene] 10). Canberra: Department of Health, 1965; pp 44-48.
- 29. Senate Community Affairs References Committee. Growing evidence of an emerging tick-borne disease that causes a Lyme like illness for many Australian patients [webpage]. http://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Lyme-like_Illness (accessed July 2016).
- 30. Vaughter S, Vaughter J. When ALS is Lyme. Examining the link between ALS and neuroborreliosis [e-book]. Revised June 2015. http://www.als-cure.com/ALS.pdf (accessed July 2016).
- 31. Fritzsche M. Chronic Lyme borreliosis at the root of multiple sclerosis — is a cure with antibiotics attainable? Med Hypotheses 2005; 64: 438-448.
- 32. Senanayake SN, Paparini A, Latimer M, et al. First report of human babesiosis in Australia. Med J Aust 2012; 196: 350-352. <MJA full text>
- 33. Gofton AW, Oskam CL, Lo N, et al. Inhibition of the endosymbiont “Candidatus Midichloria mitochondrii” during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasit Vectors 2015; 8: 1.
- 34. Collignon PJ. Intravascular catheter associated sepsis: a common problem. The Australian Study on Intravascular Catheter Associated Sepsis. Med J Aust 1994; 161: 374-378.
- 35. Ong C, Roberts J, Collignon P. Long-term survival outcome following Staphylococcus aureus bacteraemia. Healthcare Infection 2013; 18: 102-109.
- 36. Patel R, Grogg KL, Edwards WD, et al. Death from inappropriate therapy for Lyme disease. Clin Infect Dis 2000; 31: 1107-1109.
- 37. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med 2016; 374: 1209-1220.
- 38. Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med 2001; 345: 85-92.
- 39. Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology 2003; 60: 1923-1930.
- 40. Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008; 70: 992-1003.
- 41. Toyne H, Webber C, Collignon P, et al. Propionibacterium acnes (P. acnes) resistance and antibiotic use in patients attending Australian general practice. Aust J Dermatol 2012; 53: 106-111.
- 42. Cottle LE, Mekonnen E, Beadsworth MB, et al. Lyme disease in a British referral clinic. QJM 2012; 105: 537-543.
- 43. Parliament of Canada. Federal Framework on Lyme Disease Act Bill S.XC. 2014, c. 37). Ottawa: Ministry of Justice, 2015. http://laws-lois.justice.gc.ca/PDF/F-7.35.pdf (accessed July 2016).
- 44. Blumenthal R, Ayotte K, Gillibrand K, et al. Lyme and Tick-Borne Disease Prevention, Education, and Research Act of 2015. [United States Senate bill, introduced 4 June 2015]. https://www.congress.gov/114/bills/s1503/BILLS-114s1503is.pdf (accessed July 2016).
- 45. House of Representatives Standing Committee on Health. Report on the Inquiry into Chronic Disease Prevention and Management in Primary Health Care. Appendix A: Case study on tick-borne and Lyme-like diseases. Canberra: Commonwealth of Australia, 2016. http://www.aph.gov.au/Parliamentary_Business/Committees/House/Health/Chronic_Disease/Report (accessed July 2016).
- 46. Holmes EC. Submission to Senate Standing Committee on Community Affairs, Growing evidence of an emerging tick-borne disease that causes a Lyme like illness for many Australian patients (submission no. 546). March 2016. http://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Lyme-like_Illness/Submissions (accessed July 2016).





D Ashley Watson
Collignon and colleagues are to be commended for their thoughtful and perhaps courageous assessment of the issue of whether or not Lyme disease exists in Australia (1). As an infectious diseases physician who has been called upon at times to assess ostensible diagnoses of locally-acquired Lyme, and who has been the target of criticism (2), I would like to add three practical observations to the debate. The first is that it is fairly common knowledge that the east coast of Australia abounds in ticks. If there were Lyme disease to be diagnosed, we would certainly know of it by now. Some popular holiday areas of the south coast and northern beaches of New South Wales seem to be truly infested.(3,4) And testing by local GPs is readily available in NATA-accredited laboratories. Secondly, there has always been plenty of competition within our trade to be the first to describe the existence of novel infectious diseases in this country. My colleagues will scramble over each to have their names in print – mostly in good faith, of course. So, the assertion by some (5) that the profession’s members might be misleadingly denying the existence of a disease such as Lyme in Australia is a little hard to substantiate.
My final observation pertains to the importance of the doctor-patient relationship in this longstanding and often heated debate. All doctors should be encouraged to periodically examine their own roles in this relationship, particularly given that patients’ trust in their doctors is becoming increasingly conditional. In an age of patient-centred care and greater clinical challenges, trust must be earned through any number of professional attributes, not least of which are scientific expertise and clinical competence.
References
1. Collignon PJ, Lum GD, Robson JMB. Does Lyme disease exist in Australia? Med J Aust 2016; doi: 205. 10.5694/mja16.00824.
2. (Various authors) Lyme diagnosis dilemma. Sydney Morning Herald [Internet]. 2012 July 22: Letters to the Editor. http://www.smh.com.au/federal-politics/letters/letters-to-the-editor-20120721-22h9s.html (accessed Nov 2016).
3. Frecklington J. Coast hot spots a ticking time bomb. The Canberra Times [Internet]. 2012 September 24. ACT News. http://www.canberratimes.com.au/act-news/coast-hot-spots-a-ticking-time-bomb-20120923-26feh.html (accessed Nov 2016).
4. Eriksson B. Northern beaches hit with tick outbreak, Palm Beach, Avalon, North Narrabeen worst affected. The Daily Telegraph [Internet]. 2013 August 20. http://www.dailytelegraph.com.au/newslocal/northern-beaches/northern-beaches-hit-with-tick-outbreak-palm-beach-avalon-north-narrabeen-worst-affected/story-fngr8hax-1226700600362 (accessed Nov 2016)
5. Lyme Disease Association of Australia, 2016. http://www.lymedisease.org.au/part-2-lyme-politics/ (accessed Nov 2016).
Competing Interests: No relevant disclosures
Dr D Ashley Watson
Canberra Hospital
Patrick Charles
Competing Interests: No relevant disclosures
Assoc Prof Patrick Charles
Austin Health