The known Pre-hospital thrombolysis in patients with ST-segment elevation myocardial infarction (STEMI) has been found to be an effective strategy in randomised controlled trials.
The new Pre-hospital thrombolysis is safe and effective in the real world setting, especially in a region where transport distances to the cardiac catheterisation laboratory are great.
The implications Pre-hospital thrombolysis can be implemented in regions where timely primary percutaneous coronary intervention for STEMI patients is not available.
Primary percutaneous coronary intervention (PCI), if performed in a timely manner, is the preferred reperfusion strategy for patients with an ST-segment elevation myocardial infarction (STEMI).1 However, physicians in regions geographically isolated from a primary PCI centre are unable to perform PCI rapidly, and the optimal reperfusion strategy is therefore undefined. Recent guidelines have highlighted the importance of systems-based approaches to STEMI management, taking into account regional characteristics.2 In Australia, many patients are a long way from a primary PCI centre, and these patients usually receive thrombolysis in hospitals that are not PCI-capable. However, if reperfusion with thrombolysis fails or a contraindication prevents its application, there can be long delays in reaching a cardiac catheterisation laboratory (CCL) for PCI.3 Further, a pharmaco-invasive (PI) strategy, including routine cardiac catheterisation within 24 hours of thrombolysis, was found to achieve better outcomes than ischemia-driven angiography.4 This strategy is especially relevant in areas where geographic access and logistical delays that increase the time to treatment (eg, rural areas, large urban areas with traffic congestion) may reduce some of the benefits of primary PCI in clinical practice.5
The international Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial, in which STEMI patients who were unable to undergo primary PCI within one hour of presentation were randomised to either primary PCI or a PI strategy, confirmed that outcomes of the PI strategy at one year were not inferior to those of primary PCI.6,7 However, the STREAM trial did not include all patients who presented with STEMI; although most of those randomised underwent randomisation in the ambulance setting (81%), the patients included in the trial had presented within 3 hours of symptom onset (compared with 6–12 hours for the STEMI patients who were not included), and the median time from presentation to PCI in the PI arm was 10 hours, which may not be achievable in a real world setting. Similarly, geographical and logistical constraints that are important factors in different parts of the world, such as distance and regional resources, were not considered in the STREAM trial.
The case series described in this article was from the Hunter New England Local Health District (HNELHD), which has a complex geography and limited resources. The area it covers (130 000 km2) is comparable in size with England, but it has only one continuously operating CCL; it is located in one corner of the health district (in Newcastle), and patients with a STEMI may be as far as 600 km away (Box 1). In 2008, we implemented a system of care in HNELHD for providing rapid reperfusion to STEMI patients throughout this large district. Modelled on the Emergency Triage of Acute Myocardial Infarction study in the Northern Sydney Area Health Service, which achieved reduced delays and improved outcomes for patients with primary angioplasty,9 our system involved pre-hospital diagnosis and triage by paramedic staff, followed by their allocating the patients to either PHT or primary PCI according to their travel time to the CCL in our large regional health district. The analysis reported in this article compares the safety and effectiveness of pre-hospital assessment and allocation to pre-hospital thrombolysis (PHT) or primary angioplasty by paramedics.10
Methods
Study design
Hunter AMI was a prospective, non-randomised, consecutive, single-centre case series of STEMI patients who had been diagnosed on the basis of a pre-hospital ECG performed by paramedics. Data were collected electronically through the collaboration of the New South Wales Ambulance and the HNELHD, and data were independently analysed by the Clinical Research Design, IT and Statistical Support (CReDITSS) unit at the Hunter Medical Research Institute.
Study patients
For all patients with symptoms or signs suggesting myocardial infarction, paramedics recorded a 12-lead electrocardiogram (ECG; LIFEPAK 15 monitor system, Physio-Control Australia). Posterior and right-sided leads were not recorded by the paramedics for infero-posterior or right ventricular myocardial infarction. If the ECG indicated a possible STEMI according to the Glasgow algorithm,11,12 it was transmitted to the on-call cardiologist or cardiology advanced trainee at the John Hunter Hospital in Newcastle; the doctor and the paramedic then discussed the case, and reperfusion of the patient started. For patients who could reach a CCL within 60 minutes of first medical contact (FMC) or for whom there was any contraindication to thrombolysis (irrespective of FMC-to-CCL door time), reperfusion therapy consisted of primary PCI at the John Hunter Hospital. PHT (fibrinolysis with tenecteplase administered by paramedics) was administered by paramedics to patients for whom the FMC-to-CCL door time exceeded 60 minutes, and was followed by transfer to the PCI-capable centre (Appendix 1). All consecutive STEMI patients who had been diagnosed by pre-hospital ECG were included in our analysis.
FMC was defined as the first face-to-face contact made by paramedics with the patient. Total ischaemic time was defined as the time from symptom onset to balloon inflation for the primary PCI group, and to injection of tenecteplase for the PHT group. Hypertension was diagnosed if blood pressure greater than 140/90 mmHg was measured on two or more occasions in the hospital, or if the patient was already being treated with antihypertensive medication.13,14 Diabetes mellitus was diagnosed if two of the following applied: symptoms of hyperglycaemia; fasting sugar level > 7 mmol/L; 2-hour post-prandial sugar level > 11.1 mmol/L; HbA1c level > 6.5 mmol/mol.15 Cardiogenic shock was defined as persistent hypotension (systolic blood pressure < 90 mmHg or mean arterial pressure 30 mmHg lower than baseline).16 Left ventricular ejection fraction was recorded during the index hospitalisation by transthoracic echocardiography, using Simpson’s biplane method.
Study therapies
We present the outcomes of PHT and primary PCI performed according to our local guideline-based protocol, which includes concomitant dual antiplatelet and anticoagulant therapy. Adjunct administration of a glycoprotein IIb/IIIa antagonist was at the discretion of the intervening cardiologist. In the PHT arm, tenecteplase was administered at a weight-based dose (55 to < 60 kg, 30 mg; 60 to < 70 kg, 35 mg; 70 to < 80 kg, 40 mg; 80 to < 90 kg, 45 mg; ≥ 90 kg, 50 mg). The tenecteplase dose administered to patients aged 75 years or more has since been reduced by half. Anticoagulant therapy consisted of a 30 mg intravenous bolus of enoxaparin (omitted for patients aged 75 years or more) followed by subcutaneous injection of 1 mg/kg bodyweight (0.75 mg/kg for patients aged 75 years or more) twice each day. Antiplatelet therapy consisted of a 300 mg loading dose of clopidogrel (omitted for patients aged 75 years or more) followed by 75 mg daily, together with an initial 300 mg aspirin dose, immediately followed by 100 mg daily. Patients in the PHT arm with electrical or haemodynamic instability, ongoing ischaemic symptoms, or less than 50% ST-segment resolution within 90 minutes of thrombolysis underwent rescue PCI.
Primary endpoints
The primary efficacy endpoint was 12-month all-cause mortality. The primary safety endpoint was bleeding, categorised according to Thrombolysis in Myocardial Infarction (TIMI) study group criteria.17
Statistical analysis
Summary statistics for patient characteristics in each of the two groups are presented. Normality of distribution was assessed with the Shapiro–Wilk test. Between-group comparisons of continuous variables used independent sample t tests for parametric data and pairwise comparison median tests for non-parametric data. The Pearson χ2 test assessed differences between categorical variables. Nelson–Aalen survival distributions were estimated, and adjusted using Cox proportional hazards regression models. Predictors of 12-month mortality were assessed using logistic regression. All statistical analyses were conducted in Stata 14 (StataCorp) and SAS 9.4 (SAS Institute).
Ethics approval
The study protocol was approved by the Hunter New England Human Research Ethics Committee in August 2014 (reference, 14/06/18/5.09).
Results
From 1 August 2008 to 31 August 2013, 484 patients with STEMI diagnosed by pre-hospital ECG were allocated to PHT (150 patients) or primary PCI (334 patients) on the basis of estimated FMC-to-CCL door times or any contraindication to thrombolysis (Appendix 2). There were more patients in the primary PCI group with diabetes mellitus or hypertension, and fewer with hypercholesterolaemia (Box 2).
The median time from FMC to the start of reperfusion was 35 minutes (IQR, 28–43 min) for bolus tenecteplase and 130 minutes (IQR, 100–150 min) for balloon inflation (P = 0.001). The median time between symptom onset and treatment was correspondingly shorter in the PHT group (94 [IQR, 65–127] v 180 [IQR, 140–265] minutes) (Box 2). The median distance of patients in the PHT group from the CCL was 119 km (range, 8–483 km). The median time from symptom onset to angiography was thus longer in the PHT group than in the primary PCI group, with a delay of 4 hours for the 27% of patients who required rescue or urgent intervention, and of 49 hours for the other patients in this group. Significantly, more open vessels (TIMI flow grade of 2 or more) were found on first angiography in the PHT than in the primary PCI group (45% v 8%; P < 0.001) (Box 3).
There were three ischaemic strokes and two transient ischaemic attacks in the primary PCI group and none in the PHT group. Four of the five patients who suffered a transient ischaemic attack or stroke had atrial fibrillation, either before or detected during the admission. Bleeding was significantly more frequent in the PHT group than in the primary PCI group (9.3% v 5.1%; P = 0.001) with two (1.3%) TIMI major bleeds and one (0.7%) intracranial haemorrhage in the PHT group (Box 4).
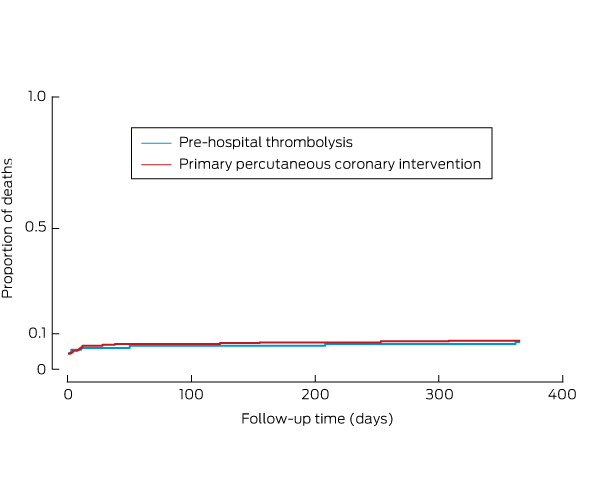
Of the total cohort of 484 patients, 34 (7.0%) died within 12 months (the primary efficacy endpoint): 10 of 150 patients (6.7%) in the PHT group and 24 of 334 (7.2%) in the primary PCI group (relative risk in the PHT group, 0.93; 95% CI, 0.45–1.9; P = 0.84) (Box 5). Anterior STEMI, cardiogenic shock, and hypertension were independent predictors of mortality for the total cohort of 484 patients in the multivariate analysis (Box 6). Age, bleeding, ejection fraction and femoral access were significant only in the univariate analysis (data not shown).
Discussion
Our study produced several important findings. First, delivery of PHT by paramedics, based on algorithm assessment of 12-lead ECGs, is feasible and safe in regional Australia. Second, 12-month mortality for patients remote from a CCL who receive PHT and are then transferred to a PCI-capable centre is similar to that for patients near the CCL who receive primary PCI.
Our practice is to administer fibrinolysis to STEMI patients who are remote from the CCL if there is no contraindication, and to then assess clinical and ECG signs of reperfusion. If they were successfully reperfused, we did not mandate immediate cardiac catheterisation but instead brought them to the CCL as soon as convenient, provided they remained clinically stable in the meantime. Those for whom reperfusion was not successful or chest pain recurred were urgently transferred to the CCL for rescue PCI.
To place our findings in context, we compared them with the outcomes of the STREAM trial.7 Our PHT group had some notable differences from the STREAM PI group: our patients were older, if not statistically significantly (62 years [SD, 13] v 60 years [SD, 12]), and a greater proportion of our patients were aged 75 years or more (18% v 14%); we also had a higher proportion of patients with cardiogenic shock (5.3% v 0.1%). Sex balance, rates of diabetes, hypertension, and prior coronary artery bypass graft surgery, and symptom onset-to-treatment times were similar for our PHT patients and the STREAM fibrinolysis group. The median time to angiography for our PHT group was 28 hours, compared with 10 hours in the STREAM fibrinolysis arm. Despite these differences, which indicate a higher risk level for our patients, survival at 12 months in the two fibrinolysis groups was identical (93.3%).
All-cause mortality at 12 months was similar for both treatment groups in our study, but this was not a randomised controlled trial, so that conclusions about treatment efficacy should not be drawn. The safety of the pre-hospital assessment and treatment, on the other hand, is reassuring. As expected, there was a significantly higher bleeding complication rate in the PHT group, but only one intracranial haemorrhage. There was a higher stroke rate in the primary PCI group; most events occurred in association with atrial fibrillation, but this finding requires further follow-up assessment of potential contributors.
The optimal ECG algorithm for the detection of STEMI is unknown. For ease of use in the field, we chose to perform standard 12-lead ECGs without right ventricular or posterior leads. For their interpretation, we use the Glasgow algorithm because of its specificity.10 It remains unknown whether the addition of right ventricular or posterior leads, or the use of another algorithm, would improve the sensitivity or specificity of detection in this setting.
The transport times for the primary PCI group in our study were considerably longer than the 60-minute FMC-to-CCL goal. The decision to administer or withhold PHT was driven mainly by the estimated FMC-to-CCL time. In our protocol, the approximate time to the CCL is estimated by the paramedics, as their knowledge of prevailing weather and traffic conditions means they are in the best position to do so. Their decision is then discussed with the cardiologist or advanced trainee. There may be some bias towards underestimating transport times, but, more importantly, there are inherent difficulties in adhering to these guidelines. First, patients who had any contraindication to fibrinolysis underwent primary PCI, regardless of the estimated FMC-to-CCL time, and this would increase overall transport times for the primary PCI group. Second, clinical factors (eg, treatment of arrhythmias, resuscitation from cardiac arrest) sometimes interfere with transport, also prolonging transfer times. It is therefore important that actual transport times are reported, not just the goal transfer times. It is hoped that knowledge of both will help when devising systems of care for other Australian regions.
The long distances to the CCL for the patients in our PHT group underscore the importance of implementing systems of care in areas with complex geography that achieve optimal outcomes for STEMI patients. The median times from symptom onset to treatment in both our treatment groups were comparable with those of the STREAM groups. This finding suggests that total ischaemic time is the interval with the greatest prognostic impact, and that we should focus on reducing this period, not than just door-to-balloon times. While medical practitioners can influence treatment intervals after FMC, community education campaigns that encourage patients to call an ambulance as soon as they have chest pain are needed for reducing the time from symptom onset to FMC.
Our study had some important limitations. First, it was a non-randomised, consecutive case series study. We present the mortality associated with two therapies, but baseline and other differences between the two groups may have contributed to the different outcomes. Second, our study was underpowered for detecting small differences in mortality, so that our results should be interpreted with caution. It is nevertheless hoped that our data can inform systems of care for patients with STEMI throughout Australia.
In conclusion, our real world experience showed that PHT delivered by paramedics followed by early transfer to a PCI-capable centre is a safe and effective reperfusion strategy for patients remote from primary PCI centres.
Box 1 – The Hunter New England Local Health District, about 600 km from north to south. The John Hunter Hospital is located in Newcastle, in the bottom right hand corner of the map*
* Adapted from: Eastwood et al (2010).8
Box 2 – Baseline characteristics of the pre-hospital thrombolysis (PHT) and primary percutaneous coronary intervention (PCI) groups
All patients |
PHT group |
Primary PCI group |
P |
||||||||||||
Total number |
484 |
150 |
334 |
||||||||||||
Age (years), mean (SD) |
64 (13) |
62 (13) |
65 (13) |
0.3 |
|||||||||||
≥ 75 years |
115 (24%) |
27 (18%) |
88 (26%) |
0.05 |
|||||||||||
Sex (males) |
365 (75%) |
114 (7%) |
251 (75%) |
0.8 |
|||||||||||
Systolic blood pressure (mmHg), mean (SD) |
130 (24) |
125 (25) |
131 (24) |
0.1 |
|||||||||||
Cardiogenic shock |
30 (6.2%) |
8 (5.3%) |
22 (6.5%) |
0.8 |
|||||||||||
Anterior STEMI |
188 (39%) |
55 (37%) |
133 (40%) |
0.5 |
|||||||||||
Cardiovascular history |
|||||||||||||||
Coronary artery disease |
96 (20%) |
28 (19%) |
68 (20%) |
0.9 |
|||||||||||
Prior coronary artery bypass graft surgery |
13 (2.7%) |
2 (1.3%) |
11 (3.3%) |
0.2 |
|||||||||||
Hypertension |
267 (55%) |
64 (43%) |
203 (61%) |
< 0.001 |
|||||||||||
Diabetes mellitus |
97 (20%) |
20 (13%) |
77 (23%) |
0.01 |
|||||||||||
Smoking |
213 (44%) |
67 (45%) |
146 (44%) |
0.8 |
|||||||||||
Hypercholesterolaemia |
198 (41%) |
76 (51%) |
122 (36%) |
0.002 |
|||||||||||
Body mass index (kg/m2), mean (SD) |
29 (5) |
28 (6) |
29 (5) |
0.08 |
|||||||||||
First medical contact to treatment (min), median (IQR) |
105 (49–140) |
35 (28–43) |
130 (100–150) |
0.001 |
|||||||||||
Symptom to treatment (min), median (IQR) |
155 (107–235) |
94 (65–127) |
180 (140–265) |
< 0.001 |
|||||||||||
Symptom to first medical contact > 3 h |
83 (17%) |
14 (9.3%) |
69 (21%) |
0.002 |
|||||||||||
Ejection fraction, mean (SD) |
48% (8)(n = 332) |
49% (10)(n = 236) |
47% (7)(n = 96) |
0.01 |
|||||||||||
Peak troponin (ng/mL), mean (SD) |
48 (32) |
44 (28) |
50 (35) |
0.04 |
|||||||||||
Haemoglobin (g/L), mean (SD) |
144 (48) |
145 (43) |
143 (56) |
0.2 |
|||||||||||
Creatinine (μmol/L), mean (SD) |
95 (31) |
89 (20) |
98 (34) |
0.006 |
|||||||||||
Length of stay (days), mean (SD) |
4 (3) |
4 (3) |
4 (3) |
1.0 |
|||||||||||
STEMI = ST-segment elevation myocardial infarction. | |||||||||||||||
Box 3 – Angiographic characteristics of the pre-hospital thrombolysis (PHT) and primary percutaneous coronary intervention (PCI) groups
PHT group |
Primary PCI group |
P |
|||||||||||||
Total number |
150 |
334 |
|||||||||||||
Coronary angiography undertaken |
138 (92%) |
334 (100%) |
< 0.001 |
||||||||||||
Rescue PCI undertaken |
37 (27%) |
NA |
— |
||||||||||||
Symptom onset to angiography (h), median (IQR) |
28 (6–70) |
3.5 (2.2–4.2) |
< 0.001 |
||||||||||||
Time to rescue PCI (h), median (IQR) |
4 (3–5) |
NA |
— |
||||||||||||
Initial TIMI flow score ≥ 2 |
67 (45%) |
27 (8.1%) |
< 0.001 |
||||||||||||
PCI/CABG undertaken |
97 (65%) |
307 (92%) |
< 0.001 |
||||||||||||
Femoral access |
52 (38%) |
153 (46%) |
0.08 |
||||||||||||
IIb/IIIa antagonist administered |
7 (4.7%) |
151 (45.2%) |
0.004 |
||||||||||||
CABG = coronary artery bypass graft surgery; NA = not applicable; TIMI = Thrombolysis in Myocardial Infarction study group. | |||||||||||||||
Box 4 – Primary efficacy and safety outcomes for the pre-hospital thrombolysis (PHT) and primary percutaneous coronary intervention (PCI) groups
PHT group |
Primary PCI group |
P |
|||||||||||||
Primary efficacy outcome |
|||||||||||||||
12-month all-cause mortality |
10 (6.7%) |
24 (7.2%) |
0.84 |
||||||||||||
Primary safety outcomes |
|||||||||||||||
Intracranial haemorrhage |
1 (0.7%) |
0 |
— |
||||||||||||
Total bleeding (TIMI bleeding criteria) |
14 (9.3%) |
17 (5.1%) |
0.001 |
||||||||||||
TIMI major bleeding |
2 (14%*) |
0 |
— |
||||||||||||
TIMI minor bleeding |
5 (36%*) |
9 (53%*) |
0.005 |
||||||||||||
TIMI minimal bleeding |
7 (50%*) |
8 (47%*) |
0.5 |
||||||||||||
TIMI = Thrombolysis in Myocardial Infarction study group. * Percentage of all bleeding. | |||||||||||||||
Box 5 – Nelson–Aalen cumulative hazard estimates for patients receiving pre-hospital thrombolysis or primary percutaneous coronary intervention
Box 6 – Independent predictors of mortality in 484 patients with ST-segment elevation myocardial infarction (multivariate analysis)
Odds ratio (95% CI) |
P |
||||||||||||||
Anterior STEMI |
2.4 (1.2–4.8) |
0.01 |
|||||||||||||
Cardiogenic shock |
8.5 (3.4–21.3) |
< 0.001 |
|||||||||||||
Hypertension |
4.2 (1.8–10) |
0.001 |
|||||||||||||
STEMI = ST-segment elevation myocardial infarction. | |||||||||||||||
Received 9 December 2015, accepted 10 June 2016
- Arshad A Khan1
- Trent Williams1
- Lindsay Savage1
- Paul Stewart2
- Asma Ashraf3
- Allan J Davies1
- Steven Faddy2
- John Attia1
- Christopher Oldmeadow3
- Rohan Bhagwandeen1
- Peter J Fletcher1,3
- Andrew J Boyle1,3
- 1 John Hunter Hospital, Newcastle, NSW
- 2 NSW Ambulance, Sydney, NSW
- 3 Hunter Medical Research Institute, University of Newcastle, Newcastle
The authors gratefully acknowledge the assistance of New South Wales ambulance paramedics in administering this protocol and in collecting the data.
Andrew Boyle and Peter Fletcher have received honoraria from Boehringer Ingelheim.
- 1. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362-e425.
- 2. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569-2619.
- 3. Gershlick AH, Stephens-Lloyd A, Hughes S, et al. Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction. N Engl J Med 2005; 353: 2758-2768.
- 4. Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al. Routine invasive strategy within 24 hours of thrombolysis versus ischaemia-guided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364: 1045-1053.
- 5. Danchin N, Puymirat E, Steg PG, et al. Five-year survival in patients with ST segment-elevation myocardial infarction according to modalities of reperfusion therapy: the French Registry on Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) 2005 Cohort. Circulation 2014; 129: 1629-1636.
- 6. Armstrong PW, Gerslick AH, Goldstein P, et al. Fibrinolysis or primary PCI in ST segment elevation myocardial infarction. N Engl J Med 2013; 368: 1379-1387.
- 7. Sinnaeve PR, Armstrong PW, Gershlick AH, et al. ST-segment-elevation myocardial infarction patients randomized to a pharmaco-invasive strategy or primary percutaneous coronary intervention: Strategic Reperfusion Early After Myocardial Infarction (STREAM) 1-year mortality follow-up. Circulation 2014; 130: 1139-1145.
- 8. Eastwood K, Durrheim D, Merritt T, et al. Field exercises are useful for improving public health emergency responses. Western Pac Surveill Response J 2010; 1: 12-18.
- 9. Carstensen S, Nelson GC, Hansen PS, et al. Field triage to primary angioplasty combined with emergency department bypass reduces treatment delays and is associated with improved outcome. Eur Heart J 2007; 28: 2313-2319.
- 10. Harper RW, Lefkovits J. Prehospital thrombolysis followed by early angiography and percutaneous coronary intervention where appropriate — an underused strategy for the management of STEMI. Med J Aust 2010; 193: 234-237. <MJA full text>
- 11. Macfarlane PW, Browne D, Devine B, et al. Modification of ACC/ESC criteria for acute myocardial infarction. J Electrocardiol 2004; 37: 98-103.
- 12. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part VI: acute ischemia/infarction. J Am Coll Cardiol 2009; 53: 1003-1011.
- 13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507-520.
- 14. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206-1252.
- 15. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33 Suppl 1: S62-S69.
- 16. Reynolds HR, Hochman JS. Cardiogenic shock current concepts and improving outcomes. Circulation 2008; 117: 686-697.
- 17. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation 2011; 123: 2736-2747.





Abstract
Objective: The system of care in the Hunter New England Local Health District for patients with ST-segment elevation myocardial infarction (STEMI) foresees pre-hospital thrombolysis (PHT) administered by paramedics to patients more than 60 minutes from the cardiac catheterisation laboratory (CCL), and primary percutaneous coronary intervention (PCI) at the CCL for others. We assessed the safety and effectiveness of the pre-hospital diagnosis strategy, which allocates patients to PHT or primary PCI according to travel time to the CCL.
Design, setting and participants: Prospective, non-randomised, consecutive, single-centre case series of STEMI patients diagnosed on the basis of a pre-hospital electrocardiogram (ECG), from August 2008 to August 2013. All patients were treated at the tertiary referral hospital (John Hunter Hospital, Newcastle).
Main outcome measures: The primary efficacy endpoint was all-cause mortality at 12 months; the primary safety endpoint was bleeding.
Results: STEMI was diagnosed in 484 patients on the basis of pre-hospital ECG; 150 were administered PHT and 334 underwent primary PCI. The median time from first medical contact (FMC) to PHT was 35 minutes (IQR, 28–43 min) and to balloon inflation 130 minutes (IQR, 100–150 min). In the PHT group, 37 patients (27%) needed rescue PCI (median time, 4 h; IQR, 3–5 h). The 12-month all-cause mortality rate was 7.0% (PHT, 6.7%; PCI, 7.2%). The incidence of major bleeding (TIMI criteria) in the PHT group was 1.3%; no patients in the primary PCI group experienced major bleeding.
Conclusion: PHT can be delivered safely by paramedical staff in regional and rural Australia with good clinical outcomes.