Abstract
Objectives: To examine trends in codeine-related mortality rates in Australia, and the clinical and toxicological characteristics of codeine-related deaths.
Design and setting: Analysis of prospectively collected data from the National Coronial Information System on deaths where codeine toxicity was determined to be an underlying or contributory cause of death. The study period was 2000–2013.
Main outcome measures: Population-adjusted numbers (per million persons) of (1) codeine-related deaths, classified by intent (accidental or intentional); and (2) heroin- and Schedule 8 opioid-related deaths (as a comparator).
Results: The overall rate of codeine-related deaths increased from 3.5 per million in 2000 to 8.7 per million in 2009. Deaths attributed to accidental overdoses were more common (48.8%) than intentional deaths (34.7%), and their proportion increased during the study period. High rates of prior comorbid mental health (53.6%), substance use (36.1%) and chronic pain (35.8%) problems were recorded for these deaths. For every two Schedule 8 opioid-related deaths in 2009, there was one codeine-related death. Most codeine-related deaths (83.7%) were the result of multiple drug toxicity.
Conclusions: Codeine-related deaths (with and without other drug toxicity) are increasing as the consumption of codeine-based products increases. Educational messages are needed to better inform the public about the potential harms of chronic codeine use, especially in the context of polypharmacy.
Research has found that increased prescribing of opioid analgesics during the past decade has resulted in rises in mortality caused by overdose in many developed countries.1-4 One opioid analgesic that has not received much attention is codeine, which is often used in the belief that it is a weaker opioid, less likely to cause dependence and fatal overdose than morphine, for example. It is important to test this assumption, as more codeine is consumed in the United Kingdom, Canada and Australia than any other opioid.5 Codeine is also one of the most accessible opioids, available without prescription (over-the-counter) in the UK, Canada, France, New Zealand and Australia.6
Concerns have been raised in a number of countries about the adverse consequences of codeine use. Use among children has been limited in several countries, and there have been calls to completely remove codeine from the market.7,8
There are documented risks associated with prolonged codeine use. It can produce tolerance, which may lead to escalating doses and dependence, particularly among patients whose pain is not well managed.6,9-11 Risks are also associated with products that combine codeine with other analgesics. Prolonged use of high-dose codeine–ibuprofen combinations has been linked with gastrointestinal disease and renal failure,12-14 while paracetamol–codeine combinations have been linked with hepatotoxicity.15,16 Dose escalation, which commonly occurs in the course of long-term codeine use, also increases the risks of side effects associated with ibuprofen and paracetamol.6
There are also concerns about the variability with which codeine is metabolised. Codeine is converted to morphine in the liver by the enzyme cytochrome P450 2D6.8,17 This enzyme is subject to genetic variation. Some individuals (about 7%–10% of the European white population) are poor metabolisers, meaning that little codeine is converted into morphine and analgesia is ineffective. About 5% of the white population are ultrarapid metabolisers, meaning there is greater conversion to morphine and increased risk of adverse events, such as respiratory depression and fatal overdose.11
Codeine-related mortality has increased in a number of countries, including the UK,18 and case series of codeine deaths have been reported in the United States,19 the UK and Australia.20,21 Case studies in the US have also documented deaths caused by the postoperative use of codeine in children.19
Analysis of mortality data provides an opportunity to systematically examine the characteristics of codeine users who experience the most severe adverse consequence, a fatal overdose. In this article, we present data on codeine-related mortality in Australia, examining:
rates of accidental and intentional codeine-related mortality;
rates of heroin- and Schedule 8 opioid-related mortality;
demographic, mental and physical health characteristics of codeine-related mortality cases; and
the circumstances of these deaths, including the presence of other drugs and the origin of codeine products consumed before death (prescribed or obtained over the counter).
Methods
Coding of deaths
We searched the National Coronial Information System (NCIS) for deaths during the period 2001–2013 in which codeine toxicity or overdose was recorded as a direct or a contributory cause of death. The NCIS is a centralised online record of deaths reported to the coroner. The NCIS contains coronial files from all states and territories in Australia and from New Zealand, but this article reviews only deaths in Australia, where routine toxicological screening is conducted after most drug-related deaths. Findings on the role of particular substances in these deaths are made by a coroner, often on the advice of forensic toxicologists.
Searches were conducted using the category “pharmaceutical substance for human use”, leaving the descriptor field for these substances blank, or specifying the subcategory as “analgesic, antipyretic, antirheumatic”, and then further specifying “codeine”. Keyword searches were also conducted in findings documents, yielding additional cases.
The focus of our study concerned codeine-related deaths, and the methods outlined in Appendix 1 were used to extract cases of interest. Appendix 2 outlines the methods used to distinguish between deaths involving codeine only, and those involving both codeine and morphine. It can be technically difficult to distinguish between deaths caused by codeine, heroin or morphine because of the manner in which these drugs are metabolised. Codeine is metabolised to morphine, and codeine can also be produced as a metabolite of heroin. Cases were removed from the dataset in which there was clear evidence that heroin had been consumed before death (eg, the detection of 6-monacetylmorphine, or eyewitness reports of heroin use), as were those in which there was no evidence of codeine consumption.
Codeine-related deaths were therefore defined as deaths where codeine was detected and codeine toxicity contributed to death, including deaths attributed to multiple drug toxicity.
During the period 2000–2013, we identified a total of 1444 deaths in which codeine toxicity was a contributory cause of death. Seven deaths were excluded because, on closer examination, they were not clearly drug-related (eg, the cause of death was recorded as gunshot wound, with codeine toxicity as a contributory cause), or because codeine was recorded in the toxicological report but was not considered to be a contributory cause of death. The remaining 1437 cases were included in our analysis.
Coding of additional variables
Several variables of interest were not consistently coded in the NCIS because of differing jurisdictional procedures. These included whether the deceased had a recorded history of injecting drug use, chronic pain, mental health problems or substance use problems (including misuse and dependence). In addition, where possible, we recorded the brand name of the codeine consumed (eg, Panadeine forte, containing 500 mg paracetamol and 30 mg codeine phosphate; Panadeine, containing 500 mg paracetamol and 8 mg codeine phosphate; Nurofen Plus, containing 200 mg ibuprofen and 12.8 mg codeine phosphate), and these were separated into over-the-counter (OTC) and prescription products. All these variables were coded using information contained in police, autopsy and findings reports.
Data analysis
Analyses were conducted with SPSS version 22 (IBM). Numbers of deaths per million population were calculated using Australian Bureau of Statistics estimates of the resident population for 30 June of each year. Differences in the intent characteristics of cases (accidental, intentional, not determined) were analysed by multinomial logistic regression.
We present data on the characteristics of cases as well as on whether the deaths were intentional or accidental for the period 2000–2013. We present trends in codeine-related deaths by intent and as rates per million population for the period 2000–2009 only. As data for 2010–2013 were likely to be incomplete (some cases would not yet have been finalised on the NCIS), trends for the segment 2010–2013 could not be reliably determined.
Trends over time were tested using Poisson regression modelling. The outcome variable for the model was intent (accidental or intentional). Findings were considered significant when P < 0.05.
In order to place codeine-related deaths into the broader context of opioid-related deaths, NCIS data for other opioid-related deaths are also presented here; that is, where heroin, Schedule 8 opioids (including buprenorphine, fentanyl, methadone, morphine or oxycodone) or multiple opioid toxicity were identified as underlying or contributory causes of death.
Ethics approval
Ethics approval for analysis of NCIS data was granted by the University of New South Wales Human Research Ethics Committee (HC 13081) and the Department of Justice Human Research Ethics Committee (CF/12/22067).
Results
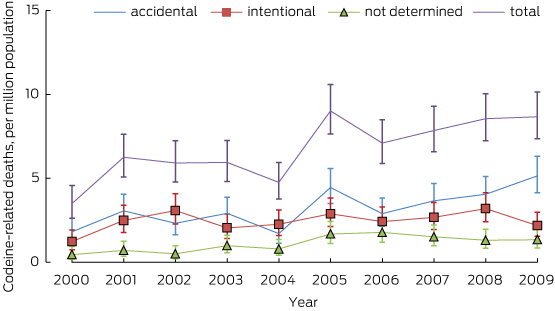
Poisson regression modelling indicated that the rate of all codeine-related deaths increased during the period 2000–2009 by 0.5 deaths per million persons per year, from 3.5 deaths per million in 2000 to 8.7 per million in 2009 (P < 0.01). The rate of deaths due to accidental codeine overdose also increased significantly, with a 9.3% (95% CI, 5.4%–13.5%) increase recorded each year, from 1.8 to 5.1 deaths per million persons (P < 0.001; Box 1). There was no significant trend in intentional codeine overdose deaths during 2000–2009.
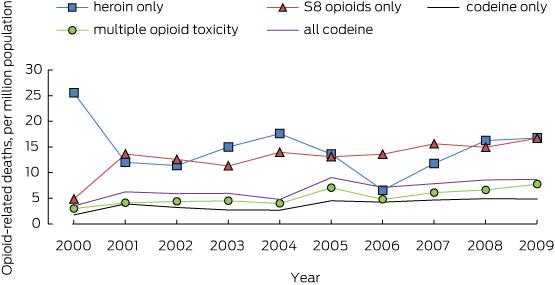
Deaths attributed to heroin, Schedule 8 opioids, and multiple opioid toxicity were more common than codeine-related deaths (Box 2). The rate was highest for heroin- and Schedule 8 opioid-related deaths, at about 16 deaths per million persons for each in 2009.
Most codeine-related deaths during 2000–2013 (1201, 83.7%) were attributed to multiple drug toxicity. A small proportion (113, 7.8%) were specifically attributed to codeine toxicity (105, codeine toxicity alone; six, combined carbon monoxide and codeine toxicity; two, combined alcohol and codeine toxicity). The remaining 123 deaths (8.5%) were attributed to other underlying causes, such as coronary heart disease, cardiovascular conditions and other drug toxicity (Box 3). The rate of increase in the number of deaths specifically attributed to codeine was greater than the rate of increase in the number of deaths attributed to multiple drug toxicity (data not shown).
The numbers of codeine-related deaths among men and women were similar, and the mean age at death was 45 years (Box 3). Just under half the deaths (701, 48.8%) were attributed to accidental overdose and a third (499, 34.7%) to intentional self-harm. Intent was not determined in the remaining 237 deaths (16.5%).
More than half (53.6%) of the cases of codeine-related death included a history of mental health problems, 36.1% a history of substance use problems (including misuse and dependence), 35.8% a history of chronic pain, 16.3% a history of injecting drug use, and 2.7% a history of cancer.
In 59.9% of cases (861), there was no information about whether the codeine consumed before death was prescribed or obtained over the counter. Where the name or specific details of the codeine product were available, a prescription codeine product (most commonly Panadeine forte) was recorded in 59.9% of cases (343 of 572), and OTC codeine products were recorded in the remaining 229 cases.
Deaths caused by accidental versus intentional overdose
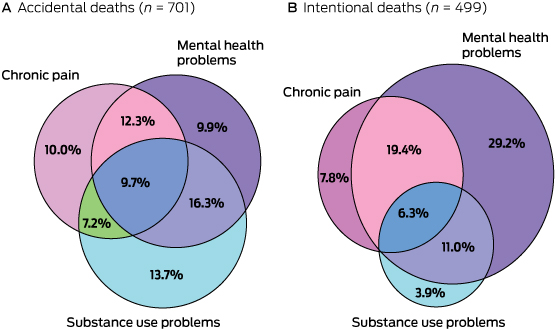
Those who had intentionally overdosed were more likely to be older, female and have a history of mental health problems, and were less likely to have a history of substance use problems, chronic pain or injecting drug use (Box 3). Mental health problems were common in those involved in intentional deaths, and the combination of chronic pain and mental health problems were recorded for 25.7% of these cases (Box 4).
A range of other drugs were detected during the toxicological investigation of these deaths. The most common were benzodiazepines (56.1%), paracetamol, ibuprofen or doxylamine (54.6%; indicative of the use of combination codeine products), antidepressants (37.8%) and alcohol (25.9%). Combination codeine products were significantly more likely to be recorded in intentional overdoses (65.1%) than in accidental overdoses (48.6%; odds ratio [OR], 1.9; 95% CI, 1.5–2.5). It was significantly less likely that pharmaceutical opioids (17.6%; OR, 0.4; 95% CI, 0.3–0.5) or illicit drugs (6.8%; OR, 0.4; 95% CI, 0.2–0.5) were detected in intentional overdoses than in those who accidentally overdosed (35.5% and 16.4%, respectively) (Box 5).
Discussion
The rate of codeine-related deaths increased significantly between 2000 and 2009, from 3.5 to 8.7 deaths per million population. The increase was primarily driven by an increase in accidental deaths. A potential driver may have been the introduction in Australia of OTC products containing larger amounts of codeine, including codeine combined with ibuprofen.22,23 The rate of codeine-related deaths was around half that of deaths attributed to heroin and Schedule 8 opioids.
Two distinct populations were detected. Those who had intentionally overdosed were more likely to be older, female and have a history of mental health problems; those who had accidentally overdosed were more likely to have a history of substance use problems, chronic pain and injecting drug use. These patterns suggest that, in the accidental deaths, there may be evidence of: (a) codeine being used to top up prescribed pain medication; (b) dose escalation of codeine; and (c) the development of codeine dependence. These characteristics highlight a complex patient population in need of specialist services.
In cases where data were available, most deaths involved people who had been prescribed codeine products, although a significant minority (40.0%) had used OTC codeine products. The investigation of trends in the sales of OTC codeine as well as of codeine prescribing patterns is warranted, and will assist understanding patterns of codeine consumption and related harms in Australia.
Clinical implications
Our findings suggest the need for different public health and clinical strategies to prevent fatal intentional and accidental codeine overdoses. Suicide prevention strategies are needed to reduce intentional overdose deaths. A meta-analysis of suicide prevention strategies24 indicated that educating general practitioners has significantly reduced suicide rates in a number of countries. An increased focus on screening for depression and suicide risk is important when prescribing codeine in primary care encounters, which represent an important opportunity for interventions that reduce the risk of suicide.
It may not be enough to change codeine prescribing practices, given that codeine is available over the counter in Australia. Pharmacists may have an important role to play in minimising harms6 by advising consumers who purchase codeine products about the risks of chronic use and by encouraging them to seek further medical advice.
It is more challenging to reduce accidental codeine overdoses, which are more prevalent and increasing in number. The high prevalence of substance use problems among those who had accidentally overdosed suggests the need for general practitioners to screen for substance misuse history when prescribing codeine.25,26
Patient education is also essential. Patients receiving codeine should be informed about the dangers of taking too much codeine, and the increased risk of fatal overdose if they combine codeine with benzodiazepines and other pharmaceutical opioids. The risks associated with combination products containing paracetamol and codeine also need to be discussed. Evidence regarding the efficacy of patient education is limited,27 and more Australian research would be useful. Evaluative research in the US has identified strategies that have shown promise in reducing problematic patterns of pharmaceutical use and of related harms.28,29 In Australia, these strategies need to include education at the point of purchase of OTC codeine.
Finally, given the complex profile of these cases and the high prevalence of comorbid conditions, including chronic pain, mental health and substance use problems, it is clearly necessary to increase the capacity to identify high-risk patients in primary care and to respond more effectively to their needs. Increasing the capacity of specialist pain, addiction and mental health treatment services in Australia should also be a priority.
Limitations of our study
The online availability of autopsy and coroner findings and of police and toxicology reports differs greatly between jurisdictions. Given inconsistencies in reporting the recorded histories of injecting drug use, mental health problems and substance use problems, we have probably underestimated their prevalence.
Missing data on the origin of codeine products consumed prior to death (prescribed or OTC) limits inferences about the source of codeine in these deaths, and hence inferences about the extent to which the diversion of prescribed codeine contributed to these deaths. It also limits inferences that can be drawn about the likely impact of reducing OTC codeine availability on the prevalence of codeine-related mortality.
The variation in the way codeine is metabolised, which affects the risk of opioid toxicity, could not be analysed in this study. Postmortem genetic testing is not routinely undertaken in Australia.
Limitations specific to the NCIS are also important. These include case completion and availability of data, the accuracy of cause-of-death coding, and the possibility that toxicological findings may alter the determined cause of death.30 The NCIS unit undertakes quality assessment of all closed cases to ensure that the most accurate cause of death is coded.
Under-reporting of drug-related deaths is a problem. In many cases, the underlying medical cause of death is deemed to be a respiratory condition, such as asphyxia or pneumonia. We attempted to resolve this problem by searching more broadly with “codeine” and “morphine” as keywords, and by using information on contributory causes of death.
Conclusion
Increasing rates of codeine-related mortality detected by our study suggest it is important to systematically monitor codeine use and harms. Given the ready availability of OTC codeine in Australia, many people consuming codeine are not easily captured by existing monitoring systems. In addition, many may not be seen by doctors, increasing the risks of their using codeine in non-recommended ways that increase the risk of harms.
Education about the dangers of taking too much codeine and the dangers of polypharmacy is needed. Pharmacists have an important role in engaging with consumers who purchase OTC codeine products.
Finally, given the complex profile of cases reported in this study, and the high prevalence of comorbid conditions, increasing the capacity of primary care screening processes, as well as specialist pain, addiction and mental health treatment services in Australia is essential.
Box 2 – Rates of codeine-related death, per million population, compared with other opioid-related deaths, 2000–2009
Box 3 – Characteristics of the deaths in which codeine toxicity was a contributory factor, 2000–2013
Total deaths |
Accidental overdose |
Intentional overdose |
Intentional v accidental, OR (95% CI) |
Intent not determined |
Not determined v accidental, OR (95% CI) |
||||||||||
Number (%) |
1437 |
701 (48.8%) |
499 (34.7%) |
237 (16.5%) |
|||||||||||
Underlying cause of death |
|||||||||||||||
Mixed drug toxicity |
1201 (83.7%) |
617 (88.0%) |
418 (83.8%)∗ |
0.7 (0.5–0.9) |
166 (70.0%)‡ |
0.3 (0.2–0.4) |
|||||||||
Codeine toxicity |
113 (7.8%) |
54 (7.7%) |
33 (6.6%) |
0.7 (0.5–1.2) |
26 (11.0%) |
1.4 (0.8–2.2) |
|||||||||
Other |
123 (8.5%) |
30 (4.1%) |
48 (9.6%)‡ |
2.4 (1.5–3.8) |
45 (19.0%)‡ |
5.3 (3.2–8.7) |
|||||||||
Sex, female |
717 (49.9%) |
322 (45.9%) |
268 (53.7%)† |
1.3 (1.1–1.7) |
127 (53.6%)∗ |
1.4 (1.1–1.8) |
|||||||||
Mean age, years (range) |
45 (14–93) |
43 (14–92) |
46 (15–93)‡ |
1.02 (1.01–1.03) |
47 (18–92)‡ |
1.02 (1.01–1.03) |
|||||||||
Age group |
|||||||||||||||
14–19 years |
16 (1.1%) |
7 (1.0%) |
8 (1.6%) |
NA |
NP |
NA |
|||||||||
20–29 years |
154 (10.7%) |
88 (12.6%) |
50 (10.0%) |
16 (6.8%) |
|||||||||||
30–39 years |
354 (24.6%) |
202 (28.8%) |
100 (20.0%) |
52 (21.9%) |
|||||||||||
40–49 years |
415 (28.9%) |
191 (27.2%) |
149 (29.9%) |
75 (31.6%) |
|||||||||||
50–59 years |
301 (20.9%) |
143 (20.4%) |
101 (20.2%) |
57 (24.1%) |
|||||||||||
60–69 years |
125 (8.7%) |
47 (6.7%) |
56 (11.2%) |
22 (9.3%) |
|||||||||||
≥ 70 years |
72 (5.0%) |
23 (3.3%) |
35 (7.1%) |
NP |
|||||||||||
History of mental health problems |
771 (53.6%) |
337 (48.1%) |
329 (65.9%)‡ |
2.1 (1.6–2.7) |
105 (44.3%) |
0.9 (0.6–1.2) |
|||||||||
History of substance use problems |
519 (36.1%) |
329 (46.9%) |
106 (21.2%)‡ |
0.3 (0.2–0.4) |
84 (35.4%)† |
0.6 (0.4–0.8) |
|||||||||
History of chronic pain |
514 (35.8%) |
275 (39.2%) |
168 (33.7%)∗ |
0.7 (0.6–0.9) |
71 (30.0%)∗ |
0.6 (0.4–0.9) |
|||||||||
History of injecting drug use |
234 (16.3%) |
167 (23.9%) |
38 (7.6%)‡ |
0.3 (0.1–0.4) |
29 (12.2%)‡ |
0.4 (0.3–0.7) |
|||||||||
History of cancer |
39 (2.7%) |
NP |
NP |
NA |
NP |
NA |
|||||||||
OR = odds ratio; NA = not applicable; NP = not published in order to protect confidentiality (small numbers). ∗P < 0.05. †P < 0.01. ‡P < 0.001, compared with accidental overdose category. | |||||||||||||||
Box 5 – Toxicology profile of other drugs involved in codeine deaths, by intent
Drug |
Total (n = 1437) |
Accidental (n = 701) |
Intentional (n = 499) |
Intentional v accidental, OR (95% CI) |
Not determined (n = 237) |
Not determined v accidental, OR (95% CI) |
|||||||||
Benzodiazepines |
806 (56.1%) |
422 (60.2%) |
266 (53.3%)∗ |
0.7 (0.6–0.9) |
118 (49.8%)† |
0.6 (0.4–0.8) |
|||||||||
Paracetamol, ibuprofen, doxylamine§ |
785 (54.6%) |
341 (48.6%) |
325 (65.1%)‡ |
1.9 (1.5–2.5) |
119 (50.2%) |
1.1 (0.8–1.4) |
|||||||||
Antidepressants, mood stabilisers |
544 (37.8%) |
264 (37.7%) |
207 (41.5%) |
1.2 (0.9–1.5) |
73 (30.8%) |
0.7 (0.5–1.1) |
|||||||||
Alcohol |
372 (25.9%) |
186 (26.5%) |
128 (25.7%) |
1.0 (0.7–1.2) |
58 (24.5%) |
0.9 (0.6–1.3) |
|||||||||
Pharmaceutical opioids¶ |
401 (27.9%) |
249 (35.5%) |
88 (17.6%)‡ |
0.4 (0.3–0.5) |
64 (27.0%)∗ |
0.7 (0.5–0.9) |
|||||||||
Methadone |
153 (10.6%) |
115 (16.4%) |
10 (2.0%) |
28 (11.8%) |
|||||||||||
Oxycodone |
159 (11.1%) |
87 (12.4%) |
46 (9.2%) |
26 (11.0%) |
|||||||||||
Tramadol |
118 (8.2%) |
69 (9.8%) |
37 (7.4%) |
12 (5.1%) |
|||||||||||
Illicit drugs∗∗ |
167 (11.6%) |
115 (16.4%) |
34 (6.8%)‡ |
0.4 (0.2–0.5) |
18 (7.6%)† |
0.4 (0.2–0.7) |
|||||||||
Cannabis |
104 (7.2%) |
68 (9.7%) |
25 (5.0%) |
11 (4.6%) |
|||||||||||
Amphetamines |
69 (4.8%) |
49 (7.0%) |
9 (1.8%) |
11 (4.6%) |
|||||||||||
OR = odds ratio. ∗P < 0.05. †P < 0.01. ‡P < 0.001, compared with accidental overdose category. §Indicative of the use of combination codeine products. ¶Includes methadone, oxycodone, tramadol, fentanyl and buprenorphine. Fentanyl and buprenorphine numbers not shown to protect confidentiality. ∗∗Includes cannabis, amphetamines, cocaine and heroin. Heroin and cocaine not shown to protect confidentiality. | |||||||||||||||
Received 13 February 2015, accepted 31 July 2015
- Amanda Roxburgh1
- Wayne D Hall1
- Lucinda Burns1
- Jennifer Pilgrim3
- Eva Saar3
- Suzanne Nielsen1
- Louisa Degenhardt1
- 1 University of New South Wales, Sydney, NSW
- 2 University of Queensland, Brisbane, QLD
- 3 Monash University, Melbourne, VIC
- 4 National Coronial Information System, Melbourne, VIC
This article is a product of the National Illicit Drug Indicators Project, which is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvement Grants Fund. The funders had no role in the research described in this paper.
Louisa Degenhardt has received untied educational grants from Reckitt Benckiser for the postmarketing surveillance of opioid substitution therapy medications in Australia, and the development of an opioid-related behaviour scale. She has also received untied educational grants from Mundipharma to conduct postmarketing surveillance of the use of oxycodone formulations in Australia. Suzanne Nielsen has been an investigator on untied education grants from Reckitt Benckiser. These funders had no role in the research described in this article.
- 1. Stannard C. Opioids in the UK: what’s the problem? BMJ 2013; 347: f5108.
- 2. Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011; 195: 280-284. <MJA full text>
- 3. Roxburgh A, Burns L, Drummer OH, et al. Trends in fentanyl prescriptions and fentanyl-related mortality in Australia. Drug Alcohol Rev 2013; 32: 269-275.
- 4. Rintoul AC, Dobbin MD, Drummer OH, Ozanne-Smith J. Increasing deaths involving oxycodone, Victoria, Australia, 2000-09. Inj Prev 2011; 17: 254-259.
- 5. International Narcotics Control Board. Narcotic drugs: estimated world requirements for 2014; statistics for 2012. New York: United Nations, 2013. http://apps.who.int/medicinedocs/documents/s21513en/s21513en.pdf (accessed Aug 2015).
- 6. Tobin CL, Dobbin M, McAvoy B. Regulatory responses to over-the-counter codeine analgesic misuse in Australia, New Zealand and the United Kingdom. Aust N Z J Public Health 2013; 37: 483-488.
- 7. MacDonald N, MacLeod SM. Has the time come to phase out codeine? CMAJ 2010; 182: 1825.
- 8. Iedema J. Cautions with codeine. Aust Prescr 2011; 34: 133-135.
- 9. Sproule BA, Busto UE, Somer G, et al. Characteristics of dependent and nondependent regular users of codeine. J Clin Psychopharmacol 1999; 19: 367-372.
- 10. Nielsen S, Cameron J, Pahoki S. Over the counter codeine dependence. Melbourne: Turning Point Alcohol and Drug Centre, 2010. http://atdc.org.au/wp-content/uploads/2011/02/OTC_CODEINE_REPORT.pdf (accessed Aug 2015).
- 11. Carter B, Hawcutt DB, Arnott J. The restrictions to the use of codeine and dilemmas about safe alternatives. J Child Health Care 2013; 17: 335-337.
- 12. Karamatic R, Croese J, Roche E. Serious morbidity associated with misuse of over-the-counter codeine-ibuprofen analgesics. Med J Aust 2011; 195: 516. <MJA full text>
- 13. Evans C, Chalmers-Watson TA, Gearry RB. Combination NSAID-codeine preparations and gastrointestinal toxicity. N Z Med J 2010; 123: 92-93.
- 14. Frei MY, Neilsen S, Dobbin MD, Tobin CL. Serious morbidity associated with misuse of over-the-counter codeine-ibuprofen analgesics: a series of 27 cases. Med J Aust 2010; 193: 294-296. <MJA full text>
- 15. Clark R, Borirakchanyavat V, Davidson A, et al. Hepatic damage and death from overdose of paracetamol. Lancet 1973; 301: 66-70.
- 16. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364-1372.
- 17. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2012; 3: 229-243.
- 18. Office for National Statistics. Deaths related to drug poisoning in England and Wales, 2012. London: ONS, 2013. http://www.ons.gov.uk/ons/dcp171778_320841.pdf (accessed Aug 2015).
- 19. Kelly LE, Rieder M, Van Den Anker J, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics 2012; 129: e1343-1346.
- 20. Pilgrim JL, Dobbin M, Drummer OH. Fatal misuse of codeine–ibuprofen analgesics in Victoria, Australia. Med J Aust 2013; 5: 329-331. <MJA full text>
- 21. Gerostamoulos J, Burke MP, Drummer OH. Involvement of codeine in drug-related deaths. Am J Forensic Med Pathol 1996; 17: 327-335.
- 22. New drugs. Aust Prescriber 2002; 25: 94-99.
- 23. Australian Register of Therapeutic Goods. Public summary: Panadeine extra tablet blister pack. 2013. https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=101436&agid=%28PrintDetailsPublic%29&actionid=1 (accessed Aug 2015).
- 24. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA 2005; 294: 2064-2074.
- 25. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med 2005; 6: 107-112.
- 26. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10: 113-130.
- 27. Department of Health and Human Services (USA). Addressing prescription drug abuse in the United States: current activities and future opportunities. Washington DC: Department of Health and Human Services, 2013. http://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf (accessed Aug 2015).
- 28. Johnson EM, Porucznik CA, Anderson JW, Rolfs RT. State-level strategies for reducing prescription drug overdose deaths: Utah’s prescription safety program. Pain Med 2011; 12: S66-S72.
- 29. McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addict Behav 2013; 38: 2230-2235.
- 30. National Coronial Information System. Data quality and limitations [website]. 2014. http://www.ncis.org.au/data-quality/data-limitations/ (accessed Apr 2015).





Mi Strom
Not having access to the source data it's difficult to understand why the subsequent 4 years worth of data were omitted.
How can the reader determine that the growth from 2000 to 2009 weren't the statistical anomaly & that from 2010 onwards the rates returned to their previous levels.
Of the 100% of data, only 70% of the data was used to draw your conclusion. And yet there's a remaining 30% which isn't disclosed in your findings.
Competing Interests: No relevant disclosures
Mr Mi Strom
N/A
Laurence Mather
Competing Interests: No relevant disclosures
Prof Laurence Mather
Sydney Medical School
Laurence Mather
Pain, a subjective sensation, needs individual treatment, sometimes requiring flexible dosing regimens of (whatever) analgesic agent(s). Despite commonly-perceived problems of over-use and abuse of codeine specifically [1,2] and opioids generally [3], there remains the significant problem of many people receiving unsatisfactory pain management.[4,5] Most pain is self-limiting and amenable to self-medication with non-prescription analgesic agents, some containing codeine in NSAID or paracetamol combinations. This is the pharmacologically-sound principle of ‘multimodal analgesia’. Ignoring pharmacogenetic complexities about codeine metabolism in relation to analgesia, it is suffice to concur that the efficacy of low-dose codeine combinations is often equivocal in randomised trials. Nevertheless, many individuals claim greater benefit from the codeine combination than the other agent alone – and they would be the ones to know.
No drug therapy is absolutely without risks. Nonetheless, I am less pharmacologically concerned about the risk of codeine toxicity, particularly from the non-prescription analgesic combinations, than I am about gastrointestinal and/or renal toxicity in those people who will inevitably use greater doses of other analgesics such as NSAIDs or paracetamol as non-codeine replacements for pain relief. It seems increasingly likely that codeine combinations will be made prescription only, and this will be assisted by reports such as this being uncritically added to the ‘analgesic problem’ literature.
1. Scheduling proposals referred to the August 2015 meeting of the Advisory Committee on Medicines Scheduling (ACMS #15)]. Available at https://www.tga.gov.au/interim-decisions-matters-referred-expert-advisory-committee-11#codeine
2. Wiseman H. Codeine deaths need action. MJA InSight Monday, 5 October, 2015
3. Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011; 195: 280-284
4. Cousins MJ. Unrelieved pain: a major health care priority. Med J Aust 2012; 196: 373-374
5. Notcutt, W., & Gibbs, G. Inadequate pain management: myth, stigma and professional fear. Postgrad Med J 2010; 86 (1018): 453-458
Competing Interests: No relevant disclosures
Prof Laurence Mather
Sydney Medical School