Clinical record
A 62-year-old man with thrombophilia was receiving warfarin for recurrent venous and arterial thrombosis, and had a known 48 mm diameter infrarenal abdominal aortic aneurysm (AAA). He presented with collapse at home after 2 days of increasing pain in the left flank. A left-sided retroperitoneal haematoma was identified by computed tomography angiography. Increasing abdominal pain and a decline in haemoglobin levels from 125 g/L to 88 g/L made it necessary to transfer the patient urgently to theatre for exploration and open repair of a presumed ruptured AAA.
The patient had been taking 1.5 mg warfarin each day for 20 years without complication. He was known to be heterozygous for both the factor V Leiden and the prothrombin G2021A mutations. He was a current smoker with a 40-pack-year history who also had mild rheumatoid arthritis, insulin-dependent type 2 diabetes mellitus, stage 3A chronic kidney disease, moderate aortic stenosis and hypertension.
Before surgery, anticoagulation with therapeutic warfarin (international normalised ratio [INR] 2.5) was reversed according to our unit protocol with 5000 IU Prothrombinex-VF (CSL Behring Australia). A posterior rupture of the AAA was confirmed during the operation. Sodium heparin (5000 U) was administered before aortic cross-clamping, and its action was fully reversed at the end of surgery with 50 mg protamine sulphate.
Recovery was initially uneventful, and therapy with 1.5 mg warfarin was resumed on postoperative day 1, together with a renally adjusted dose of enoxaparin sodium (40 mg twice daily).
On postoperative day 5, the patient experienced increasing abdominal pain and was returned to theatre for an exploratory laparotomy; nothing significant was found. His INR was 3.1, and reversal of anticoagulation was not performed.
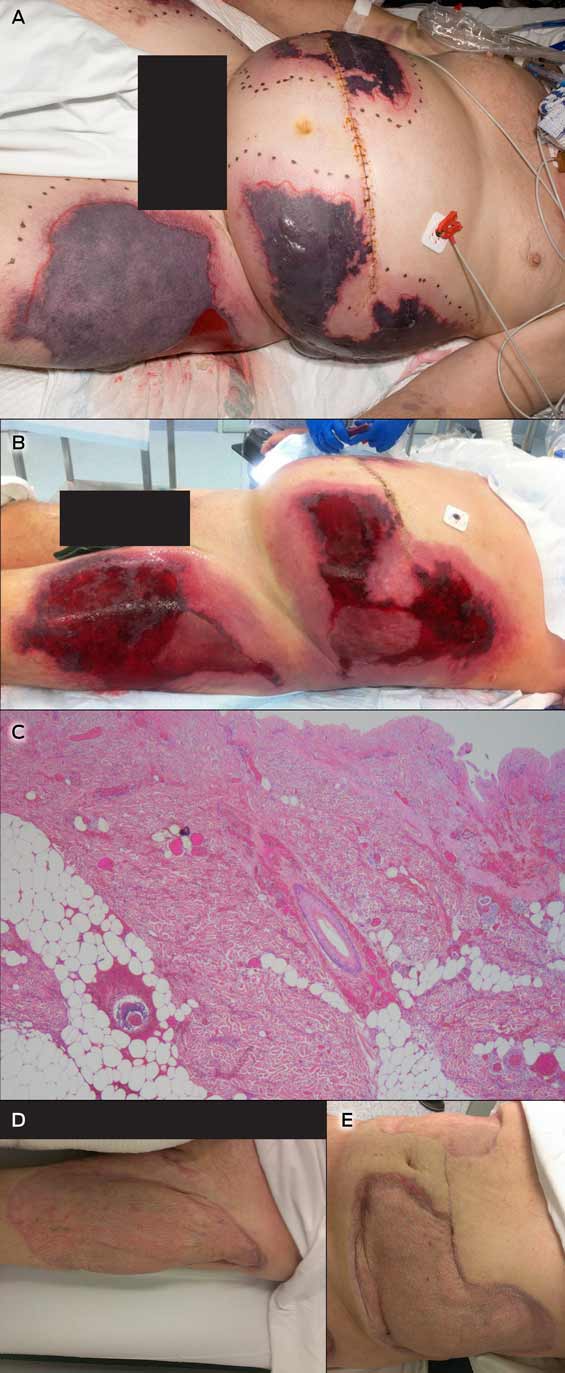
On postoperative day 8, he was transferred to the intensive care unit because of deteriorating gas exchange, hypotension and an evolving coagulopathy. Large and painful areas of skin necrosis had developed on the abdomen, flanks and thighs (Figure, A). His INR was 6.2, activated partial thromboplastin time (APTT) 64 seconds, fibrinogen levels 1.0 g/L, and platelet numbers had dropped from 265 × 109/L to 123 × 109/L.
Seven units of fresh frozen plasma and 10 units of cryoprecipitate were infused. Warfarin treatment was withdrawn, and anticoagulation therapy with intravenous heparin initiated (target APTT: 65–100 seconds) to treat the presumed warfarin-induced skin necrosis (WISN).
The results of an enzyme-linked immunosorbent assay (ELISA) test for heparin-induced thrombocytopenia were negative, as was screening for vasculitis-related antibodies. Anti-cardiolipin and anti-β2 glycoprotein I antibodies were not detected, nor was lupus anticoagulant. Protein C and S levels were low (0.45 U/mL and 0.53 U/mL, respectively).
The skin lesions continued to demarcate over the next 2 days, and were debrided on postoperative day 10 (Figure, B). Histopathological findings were consistent with WISN (Figure, C).
Anticoagulation treatment with intravenous heparin continued for 2 weeks, and was then changed to enoxaparin sodium (100 mg twice daily).
The skin lesions were regularly debrided and negative pressure dressings applied during the following months. Autologous split skin grafts were later performed with excellent results (Figure, D, E), and the patient was transferred to our rehabilitation facility on postoperative day 63. Treatment with oral rivaroxaban was initiated when he was discharged from hospital, and is to continue indefinitely at a dose of 20 mg daily.
Warfarin-induced skin necrosis (WISN) is a rare complication of a commonly used medication. The underlying mechanism is unclear, but it is thought that WISN is induced by a transient paradoxical hypercoagulable state.
Warfarin inhibits certain vitamin K-dependent factors more quickly than it does others, producing a transient imbalance in procoagulant and anticoagulant activity.1 The anticoagulant activity of protein C is rapidly reduced (within 24 hours) because of its short half-life (5–8 hours). The levels of other vitamin K-dependent coagulation factors (II, IX and X) decline at slower rates because they have longer half-lives (24–72 hours). The initial result, therefore, is a relative increase in thrombin generation and a transient hypercoagulable state that may lead to thrombotic occlusion of the microvasculature and thus tissue necrosis.
Our patient had several risk factors for WISN, including his age, obesity and a history of thrombophilia. Further, he was heterozygous for the factor V Leiden mutation (resulting in activated protein C resistance and a functional protein C deficiency) and for the prothrombin G2021A mutation (resulting in elevated prothrombin levels).2 Hypercoagulable conditions more commonly associated with WISN include deficiencies of protein C, protein S and antithrombin III. Other recognised predisposing factors include being female and being given higher loading doses of warfarin.3
The potential role of Prothrombinex-VF in the development of WISN in our patient warrants further consideration. In Australia, immediate warfarin reversal is achieved by using prothrombin complex concentrates or fresh frozen plasma. Prothrombinex-VF is the only prothrombin complex concentrate routinely used in Australia and New Zealand. It is a three-factor concentrate (prothrombin II, IX and X), including low levels of factor VII, but does not contain proteins C or S.
Our unit protocol for immediate warfarin reversal at the time of this patient's admission reflected the recommendations published in 2009 by Chiu and colleagues.4 Our patient received Prothrombinex-VF alone; neither vitamin K nor fresh frozen plasma were used during reversal. We hypothesise that protein C and S levels were low at the time of his operation, as Prothrombinex-VF does not reverse the reduction of protein C and S levels caused by warfarin. Perioperative blood loss would have reduced their levels further, and the resumption of warfarin treatment immediately after the operation would have depleted them even more. We therefore suggest that very low levels of proteins C and S, together with his pre-existing thrombophilia, are likely to have tipped the balance in favour of thrombosis.
A recent update of the consensus guidelines for warfarin reversal in Australia suggested that 5–10 mg vitamin K1 be given parenterally at the same time as Prothrombinex-VF.5 The half-lives of the infused clotting factors are similar to those of endogenous clotting factors, but the addition of vitamin K1 (as a cofactor in their synthesis) would sustain the reversal effect. It may also increase protein C and S levels, and thereby avoid a transient prothrombotic state when treatment with warfarin is resumed.
Our case highlights the importance of being aware of WISN as a rare complication of warfarin therapy. Consideration of individual patient factors, including a history of thrombosis, before initiating warfarin reversal is critical for ensuring that appropriate adjuvant therapy is provided and an optimal outcome achieved. Vitamin K1 should be administered with Prothrombinex-VF during warfarin reversal, as it sustains the reversal effect, may increase the levels of proteins C and S, and thereby avert thrombotic complications.
Lessons from practice
- Individual patient factors, including a history of thrombosis, must be considered before warfarin reversal.
- Updated consensus guidelines for warfarin reversal suggest giving vitamin K1 with Prothrombinex-VF.
- By increasing protein C and S levels, vitamin K1 may prevent a transient hypercoagulable state after resuming warfarin therapy.
Provenance: Not commissioned; externally peer reviewed.
- 1. McGehee WG, Klotz TA, Epstein DJ, Rapaport SI. Coumarin necrosis associated with hereditary protein C deficiency. Ann Intern Med 1984; 101: 59-60.
- 2. Yang Y, Algazy KM. Warfarin-induced skin necrosis in a patient with a mutation of the prothrombin gene. N Engl J Med 1999; 340: 735.
- 3. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87: 266-272.
- 4. Chiu D, Grigg M, Levi E. Operating on patients with warfarin: simpler alternative approach. ANZ J Surg 2009; 79: 409-410.
- 5. Tran HA, Chunilal SD, Harper PL, et al. An update of consensus guidelines for warfarin reversal. Med J Aust 2013; 198: 198-199. <MJA full text>





No relevant disclosures.