Biologically, the timing and duration of sleep are regulated by two interacting systems — the homoeostatic sleep drive (process S) and the circadian system (process C).1 Process S assumes that the longer one stays awake, the more pressure there is to fall asleep. Once asleep, this pressure dissipates until a homoeostatic equilibrium is achieved. Process C regulates the timing of sleep by controlling periods of biological activity and inactivity throughout the day. These peaks and troughs in biological functioning are known as circadian rhythms and run for slightly longer than 24 hours in humans.2 Circadian rhythms are generated by the central nervous system pacemaker, the hypothalamic suprachiasmatic nucleus (SCN), sometimes called the body clock. The SCN regulates the rhythmicity of many biological processes, such as temperature and hormone release, and is responsible for synchronising these processes to each other and to the external environment.3 For all terrestrial vertebrates, evening light phase delays and morning light phase advances the biological clock. This daily resetting is how the SCN is synchronised to the 24-hour light–dark cycle and to a multitude of internal rhythms at the level of organs, tissues, cells and genes. In regard to the sleep–wake cycle, the SCN uses external cues such as light, activity and food intake (in some species) to synchronise the timing of sleep to the 24-hour cycle of the social environment. Misalignment between the circadian system and the external environment, where sleep occurs outside societal norms, leads to a circadian rhythm sleep disorder. Only delayed sleep phase disorder (DSPD) and advanced sleep phase disorder are discussed in this article; other circadian rhythm sleep disorders are described elsewhere.4
DSPD is commonly found in teenagers and young adults (average age of onset, 20 years), with the pattern developing in adolescence.4,5 Sleep onset is delayed by 3–6 hours compared with conventional times (10–11 pm).6 Once sleep is attained, it is normal in length and quality but is delayed, resulting in social and often psychological difficulties. DSPD develops due to an interaction of a delay in the intrinsic circadian rhythm and poor sleep hygiene (staying up increasingly late and often using social networking).
DSPD is relatively common in adolescents and young adults, with a prevalence of 7%–16%, and represents 10% of individuals diagnosed with chronic insomnia disorder in sleep clinics.4 Individuals with DSPD may have an extended circadian cycle of 24.75 hours or longer.3 The major sleep period is therefore delayed, with wake times set intractably late, leading to a propensity to fall asleep later and get up later until there is relative pattern.
When forced to be out of bed at conventional wake-up times, adolescents with DSPD continually experience a short sleep duration and feel permanently jetlagged. This may mask the true nature of the problem, resulting in a diagnosis of psychophysiological insomnia (PPI; also known as sleep-onset insomnia) rather than a circadian rhythm sleep disorder. Adolescents may present to a general practitioner with a history of taking “hours” to get to sleep and being extremely difficult to wake in the morning for school, university or work. They are usually accompanied by a very frustrated parent who may also describe himself or herself as a “night owl”. Exploring family history is important. Adolescents may be withdrawn, indicating an underlying depression often comorbid with DSPD.7 Anxiety symptoms may also be present. The refusal to go to bed when the rest of the family do may be misinterpreted as an adolescent behavioural issue and not a genuine sleep problem. Misunderstandings from both perspectives will negatively impact on family dynamics.
An interaction between PPI and DSPD is not uncommon in adolescence, often stemming from unrealistic parental expectations. Expecting adolescents to fall asleep immediately after being mentally active with homework in the bedroom is unrealistic. The bed in that room has become a psychological reinforcement associated with heightened mental arousal and not sleeping. Time spent on the computer in the bedroom late in the evening playing video games and social messaging has a potentially similar outcome.8
Research indicates that mean optimal daytime alertness in adolescents requires a 9-hour sleep.9 This is rarely achieved, with most students cumulatively sleep-deprived as school weekdays progress,10 negatively impacting on academic performance and psychological health,11 with the added potential of motor vehicle accidents in teenage drivers.12 Restoring the correct timing, enabling sleep for daytime functioning and safety, is paramount.
There is a paucity of studies examining treatment of DSPD. Few have examined combinations of treatments, and some have focused only on the effects of manipulating sleep timing in healthy sleepers.13,14
a chronotherapeutic regimen: changing the timing of sleep onset to progressively delay (send forward) sleep onset until it matches a more conventional time;
photic factors: bright light therapy;
chronobiotic administration: use of a phase-shifter such as melatonin;
non-photic factors and healthy sleep parameters: timing of exercise; diet; limiting the use of social media; improving mood.
Tips for assessing and treating DSPD in adolescents are provided in Box 1.
A raster plot (a graphic representation of sleep–wake patterns) or actigraphy (using a device resembling a wristwatch, which measures movement via an accelerometer to infer sleep/wakefulness from rest/activity cycles) are essential for recording sleep patterns over time.16 Once the current delayed sleep times are established, sleep/bedtime is progressively delayed (moved later and later), usually by 3 hours every 2 days or longer, until sleep onset time moves around the clock to reach the desired bedtime (around 10–11.30 pm).6 Exposure to post-sleep morning light (natural or artificial or a combination) is used to anchor sleep phase to the new, desired time. Sleep and temperature need to be in tandem to maintain this new desired sleep time (Box 2). This is a difficult treatment to implement, as it requires considerable planning, time away from usual daytime activities, specialist input and considerable family support.
For the whole of the animal kingdom, irrespective of whether the species is nocturnally or diurnally active, evening light exposure delays the clock while morning light phase advances it. Bright light therapy for DSPD must always be given after the core temperature minimum, which occurs 2–3 hours before wake-up time (Box 2). The body clock is then reset every day. At certain latitudes and seasons, natural exposure to dawn/dusk sunlight is not available and bright artificial light can be substituted to maintain a normal circadian phase. Bright light therapy at the appropriate post-sleep phase drives the sleeping times earlier, back to the desired bedtime (Box 3, B). Light intensity, spectrum, duration and distance from the source are crucial variables. Studies have shown the light intensity required to successfully advance the circadian phase is typically between 2500 and 10 000 lux.17 However, when bright light therapy is used in combination with another therapy, such as cognitive behaviour therapy, as little as 1000 lux exposure is successful.18 Retinal cells in the lower part of the eye sending information to the SCN are tuned to the blue-green end of the spectrum, and this wavelength appears more efficacious than full-spectrum lighting.19
A chronobiotic is a chemical substance capable of therapeutically re-entraining short-term dissociated or long-term desynchronised circadian rhythms, or prophylactically preventing disruption following environmental insult.20 Melatonin is the most researched chronobiotic in terrestrial non-seasonal breeding vertebrates. Human endogenous melatonin levels start to rise about 2 hours before natural sleep onset and peak about 5 hours later (Box 2).
About 40% of overnight core temperature decline during natural sleep is caused by the endogenous release of melatonin, which increases peripheral temperature.19,21 Time of day of melatonin administration is the critical variable with dose being second. Melatonin is administered at the reverse time of day to bright light therapy; ie, evening melatonin advances the sleep–wake cycle while evening light delays it (Box 3, A).
It is important to distinguish between the use of melatonin as a soporific (a weak hypnotic) for PPI15 and its use as a chronobiotic for treating DSPD. In the treatment of PPI, exogenous melatonin administration works best when taken 2 hours before the desired bedtime. When taken for DSPD, it may need to be administered 4–6 hours before the current sleep onset time and be moved progressively earlier as sleep onset moves earlier.22 A soporific effect may occur in the very early evening, with potential driving-safety consequences.
A combination of morning bright light therapy (after core temperature minimum) and evening melatonin can be an ideal treatment regimen. Compared with chronotherapy alone, this approach is more practical and manageable, owing to its shorter implementation period (10–20 days).13
Despite assurance from studies,23 there are concerns recommending administration of high doses of melatonin. Circulating endogenous melatonin levels are very high in childhood and decline precipitously at puberty, hence melatonin was speculated but not substantiated to be the pubertal hormone.24 The importance of this rapid natural decline of endogenous levels in early adolescence is unknown, and supplementing high dosages of exogenous melatonin has not been systematically researched. Although the liver is very efficient in clearing circulating levels of melatonin, with a half-life of 45–60 minutes (Box 4), a small dose of 0.3–0.5 mg was found to be as effective as 3 mg for advancing sleep onset.22,25 In the absence of data, the lowest effective dose of 1 mg is recommended (compounding pharmacies).
Prolonged-release melatonin is thought to mimic the natural endogenous release profile, phase-advance sleep and improve sleep-maintenance insomnia when used as treatment for primary insomnia in older people (> 55 years).26 Research has found the 2 mg melatonin dose subjectively improved sleep quality and morning and evening alertness in that population.26 Anecdotally, it has been used in children and adolescents; however, until there are more research data it would be prudent not to use this medication in adolescents.
Agomelatine, currently marketed as an antidepressant, is a melatonin analogue with phase-advancing properties in rodents (as S 20098)27 and humans.28 Theoretically, agomelatine may be beneficial in older adolescents who have DSPD plus depression, since circadian changes can be associated with major depression.7 It is not the absolute delay in sleep but changes to the phase angle (timing) of sleep relative to other internal changes (onset of endogenous melatonin release relative to sleep phase) that appear crucial in the onset of depression.
DSPD can be exacerbated by extrinsic factors, such as use of social media (ie, electronic devices), diet, timing of exercise, and depression and anxiety. Good sleep habits or sleep hygiene are behavioural practices that result in good sleep quality and sufficient sleep duration, and prevent daytime sleepiness.29
The alerting effect of media is strongest when light is predominantly emitted within a blue spectrum.30 Watching television, texting and using a computer or electronic tablet device are associated with delayed sleep onset and poorer sleep quality.8,31,32
Caffeine is a stimulant. The standard measure of one cup of espresso coffee (85 mg caffeine) can last 4 hours after consumption and longer.33 Energy-dense foods, such as those high in sugar content, stimulate the digestive and endocrine system, producing an alerting effect.
In general, regular exercise is a good way to promote sleep and good health. Exercise can delay sleep in young adults if undertaken at usual sleep onset time, and prolonged aerobic exercise even a few hours earlier can maintain high body temperature, increasing alertness and interfering with evening “wind down”.34
Depression is common in DSPD. If symptoms of depression are present or develop later, it is imperative to treat to reduce exacerbation or a reduction in treatment response to DSPD.7 Sleep anxiety is commonly associated with long periods of lying in bed waiting for sleep onset in DSPD.
1 Tips for assessing and treating delayed sleep phase disorder (DSPD) in adolescents presenting with severe sleep onset insomnia
Establish the patient’s full family history — ask about sleep onset difficulties in other family members
Establish whether there is a history of sleep onset difficulties as a child/adolescent. Is there a history of napping after school and difficulty getting up for school in the morning?
Establish a DSPD diagnosis based on a 2-week diary in the form of a raster plot or actigraphy
Refer the patient to a sleep clinic with circadian rhythm specialists where possible
Refer to a good reference manual — eg, Wirz-Justice et al15
Consider a chronotherapeutic regimen for school holidays if there is considerable family support
- Establish possible core temperature minimum (2–2.5 h before most usual getting up time)
- Encourage light exposure (outside or artificial light for at least 40 min) after the minimum core temperature time
- Consider carefully timed administration of a low dose of melatonin at 1 mg 4–6 hours before prescribed bedtimes
- Once desired sleep onset time is established, maintain a dose of 0.5 mg of melatonin 2 h before expected sleep onset Have realistic expectations — an individual successfully treated for DSPD is still likely to prefer a later sleep onset time
3 Phase–response curve in relation to melatonin administration and light exposure, along with how to instigate bright light therapy
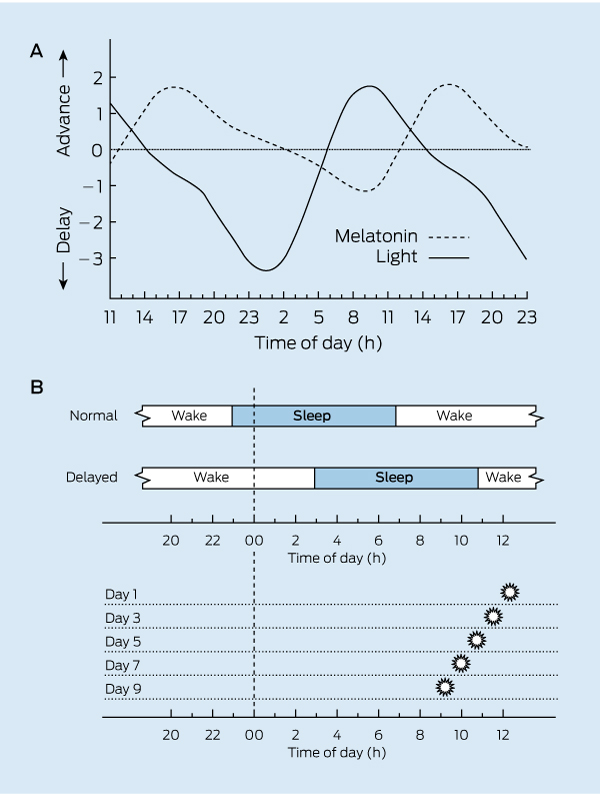
A. Phase–response curve in a normally entrained individual for melatonin (3 mg) administration over 3 consecutive days compared with bright light. Evening light phase delays the human clock while morning light phase advances. Early evening melatonin phase advances the clock while morning administration modestly delays phase. Source: Barion and Zee;13 redrawn with permission. Original data derived from Littner et al16 and Gooley.17 B. Schematic diagram of “morning” bright light therapy in a delayed sleep phase disorder patient with sleep onset at about 0300 h and natural wake-up time at 1100 h. Full-spectrum bright light exposure is moved earlier and earlier every 2 days (in this example) until the target bedtime is achieved. The decision on how often to advance light exposure is made from the advancing sleep onsets recorded daily in raster plots. If pre-sleep melatonin is administered to achieve a similar result, it would be taken earlier and earlier as sleep onset advances over successive days.
4 Natural and exogenous melatonin profiles
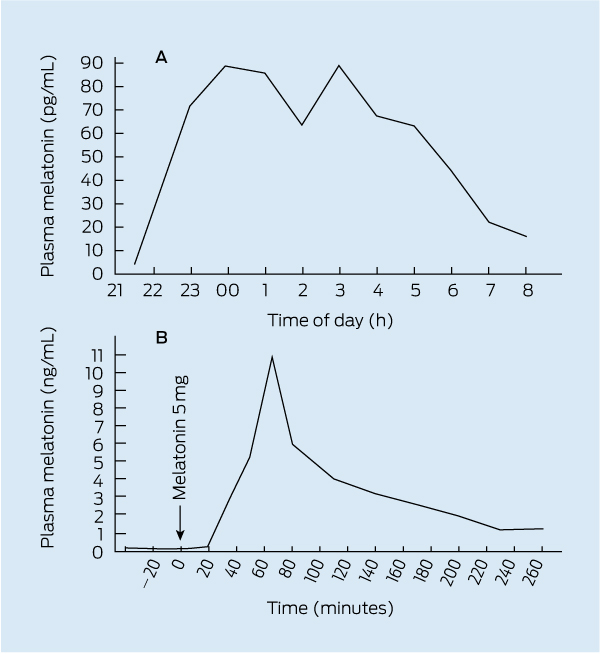
A. Endogenous plasma melatonin profile (pg/mL) of an adult male. Source: Norman TR, Armstrong SM; unpublished data, 1986; redrawn with permission. B. Plasma melatonin profile (ng/mL) of another adult male after ingestion of 5 mg melatonin capsule during daytime hours. Note the efficient clearing of circulating melatonin by the liver within a 40 min window, but despite this efficiency, the persistence of aphysiological levels (1300 pg) 4 hours postingestion. Source: Short and Armstrong;20 redrawn with permission.
Provenance: Commissioned by supplement editors; externally peer reviewed.
- Delwyn J Bartlett1
- Sarah N Biggs2
- Stuart M Armstrong3,4
- 1 Sleep and Circadian Group, Woolcock Institute of Medical Research, Sydney, NSW.
- 2 Ritchie Centre, Monash Institute of Medical Research, Melbourne, VIC.
- 3 Epworth Sleep Centre, Melbourne, VIC.
- 4 Bronowski Institute of Behavioural Neuroscience, Kyneton, VIC.
No relevant disclosures.
- 1. Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1982; 1: 195-204.
- 2. Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999; 284: 2177-2181.
- 3. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002; 418: 935-941.
- 4. American Academy of Sleep Medicine. International classification of sleep disorders. Diagnostic and coding manual. 2nd ed. Westchester, Ill: AASM, 2005.
- 5. Wyatt J, Stepanki E, Kirby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep 2006; 29: 1075-1080.
- 6. Weitzman E, Czeisler C, Coleman R, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry 1981; 38: 737-746.
- 7. Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep 2009; 11: 459-465.
- 8. Li S, Jin X, Wu S, et al. The impact of media use on sleep patterns and sleep disorders among school-aged children in China. Sleep 2007; 30: 361-367.
- 9. Carskadon MA, Wolfson AR, Acebo C, et al. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep 1998; 21: 871-881.
- 10. Carskadon MA, Harvey K, Duke P, et al. Pubertal changes in daytime sleepiness. Sleep 1980; 2: 453-460.
- 11. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev 1998; 69: 875-887.
- 12. Pack AI, Pack AM, Rodgman E, et al. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev 1995; 27: 769-775.
- 13. Barion A, Zee P. A clinical approach to circadian rhythm sleep disorders. Sleep Med 2007; 8: 566-577.
- 14. Burke T, Markwald R, Chinoy E, et al. Combination of light and melatonin time cues for phase advancing the human circadian clock. Sleep 2013. In press.
- 15. Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders. A clinican’s manual for light and wake therapy. Basel: Karger, 2009.
- 16. Littner M, Kushida C, Anderson W, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 2003; 26: 337-341.
- 17. Gooley J. Treatment of circadian rhythm sleep disorders with light. Ann Acad Med Singapore 2008; 37: 669-676.
- 18. Gradisar M, Dohnt H, Gardener G, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep 2011; 34: 1671-1680.
- 19. Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int 2001; 18: 801-808.
- 20. Short R, Armstrong S. Method for minimizing disturbances in circadian rhythms of bodily performance and function. United States Patent 4660723. 1986.
- 21. Krauchi K, Cajochen C, Mori D, et al. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of body temperature. Am J Physiol 1997; 272: R1178-R1188.
- 22. Mundey K, Benloucif S, Harsanyi K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep 2005; 28: 1271-1278.
- 23. Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG. Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res 2009; 47: 1-7.
- 24. Waldhauser F, Weiszenbacher G, Frisch H, et al. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet 1984; 1: 362-365.
- 25. Burgess H, Revell V, Molina T, Eastman C. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3 mg. J Clin Endocrinol Metab 2010; 95: 3325-3331.
- 26. Lemoine P, Nir T, Laudon M, Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res 2007; 16: 372-380.
- 27. Armstrong SM, McNulty OM, Guardiola-Lemaitre B, Redman JR. Successful use of S20098 and melatonin in an animal model of delayed sleep-phase syndrome (DSPS). Pharmacol Biochem Behav 1993; 46: 45-49.
- 28. Ferguson SA, Rajaratnam SM, Dawson D. Melatonin agonists and insomnia. Expert Rev Neurother 2010; 10: 305-318.
- 29. Mindell JA, Meltzer LJ, Carskadon MA, Chervin RD. Developmental aspects of sleep hygiene: findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med 2009; 10: 771-779.
- 30. Ruger M, St Hilaire MA, Brainard GC, et al. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J Physiol 2013; 591 (Pt 1): 353-363.
- 31. Van den Bulck J. Text messaging as a cause of sleep interruption in adolescents, evidence from a cross-sectional study. J Sleep Res 2003; 12: 263.
- 32. Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep 2004; 27: 101-104.
- 33. Kamimori GH, Karyekar CS, Otterstetter R, et al. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm 2002; 234: 159-167.
- 34. Baehr EK, Eastman CI, Revelle W, et al. Circadian phase-shifting effects of nocturnal exercise in older compared with young adults. Am J Physiol 2003; 284: R1542-R1550.





Summary
Delayed sleep phase disorder (DSPD) — a circadian rhythm sleep disorder — is most commonly seen in adolescents.
The differential diagnosis between DSPD and conventional psychophysiological insomnia is important for correct therapeutic intervention.
Adolescent DSPD sleep duration is commonly 9 hours or more.
Depression may be comorbid with DSPD.
DSPD has a negative impact on adolescent academic performance.
DSPD treatments include bright light therapy, chronotherapeutic regimens, and administration of melatonin as a chronobiotic (as distinct from a soporific).
Attention to non-photic and extrinsic factors including healthy sleep parameters is also important to enable better sleep and mood outcomes in adolescents.