Clinical record
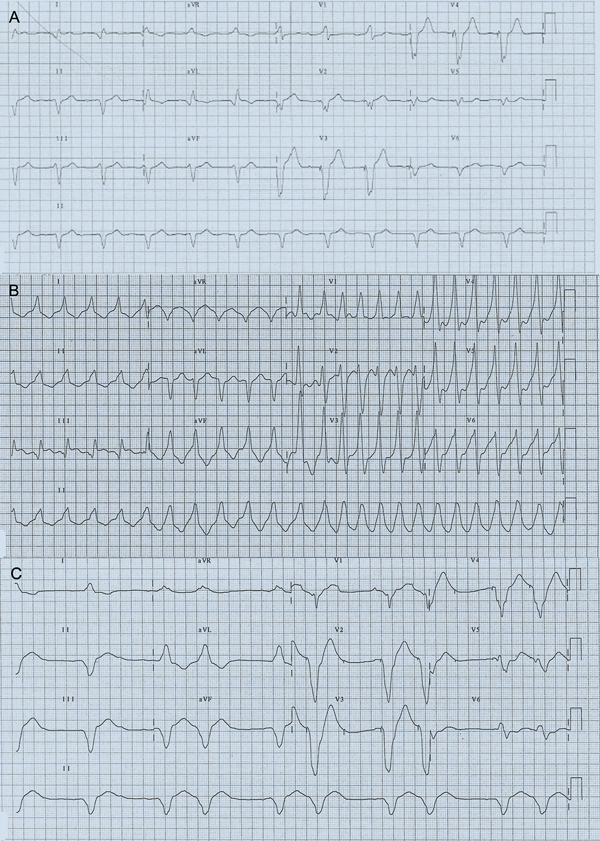
The next day, he returned to his local district hospital after experiencing another ICD shock. He was found to be in his usual paced rhythm (Box 1, A), with frequent runs of non-sustained monomorphic VT (Box 1, B). Given that he was already on amiodarone, a lignocaine infusion (maintenance rate, 2 mg/min) was added. This resulted in suppression of VT, but was complicated by confusion and perioral paraesthesia, which persisted despite reduction of infusion rate. Lignocaine was stopped after 24 hours, but this was followed soon afterwards by a VT storm.
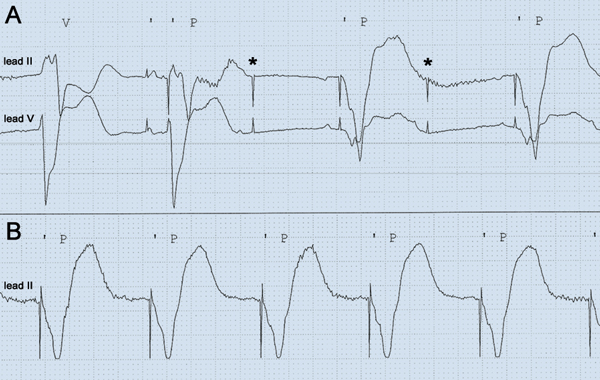
Due to the previous success of lignocaine (a class IB antiarrhythmic drug) in controlling VT, trial of another agent in this class was warranted. Mexiletine or tocainide were not readily available; however, we noted that phenytoin had class IB activity and was readily available on the ward. Phenytoin was initially administered as a loading dose (15 mg/kg, 1 g/h) and was immediately successful in suppressing the VT storm. However, at the end of the infusion, the patient developed nystagmus, drowsiness and hypotension. QRS duration lengthened (Box 1, C). Bradycardia (heart rate, 35–45 beats/min) resulting from intermittent loss of capture of right ventricular pacing was also noted on continuous telemetry (Box 2, A). This was confirmed on pacemaker–ICD interrogation. The right ventricular pacing threshold had increased from 1.4 V at 0.4 ms before phenytoin to 3.0 V at 0.4 ms after phenytoin (Box 3). Previous right ventricular pacing output had been set at 2.8 V at 0.4 ms, below the new threshold, which explained the frequent loss of capture. The pacing amplitude was increased to accommodate the effects of phenytoin loading and ongoing maintenance therapy (Box 2, B). The defibrillation threshold (21 J at time of implantation) was not initially rechecked, but due to the concern that this may also have been increased by phenytoin, the shock setting was increased to the maximum (41 J). Regular oral phenytoin (100 mg three times daily) was commenced, with trough levels confirming that the patient was in the therapeutic range (Day 7 after commencement, 52 µmol/L; Day 14 after commencement, 70 µmol/L; reference interval, 40–100 µmol/L).
Repeat pacemaker–ICD interrogation performed 9 days after phenytoin commencement demonstrated sustained suppression of ventricular ectopy, with only one further episode of VT, which was successfully terminated by antitachycardia pacing. The pacing thresholds stabilised at a higher level 12 days after phenytoin commencement (Box 3) and remained stable thereafter. A defibrillation threshold test performed 46 days after phenytoin commencement showed no change to the threshold.
Phenytoin, a widely used anticonvulsant, has class IB antiarrhythmic drug (AAD) properties, and is a potential option for patients with refractory ventricular arrhythmia when other agents are contraindicated or unavailable. In patients with frequent ventricular arrhythmia, AADs are often used to reduce the frequency of arrhythmia and implantable cardioverter defibrillator (ICD) shocks. Amiodarone is often used as first-line therapy. Although class I AADs are generally not prescribed for patients with ischaemic heart disease or cardiomyopathy, they are occasionally used in combination with amiodarone in patients who are refractory to amiodarone monotherapy, especially those with ICDs, as this provides some protection against potential proarrhythmic side effects.1
Phenytoin has class IB antiarrhythmic properties due to its effects on sodium channels in cardiac myocyte and Purkinje fibre cell membranes.2 It reduces the maximum rate of depolarisation of the cardiac action potential and increases the effective refractory period. Therefore, it is particularly effective in inhibiting ventricular ectopy, especially in an ischaemic or damaged myocardium.3 Although used as an AAD from the 1950s to the 1970s,4-6 phenytoin has become obsolete in recent decades due to the arrival of newer and less toxic agents. Recent case reports have documented its successful use in controlling idiopathic ventricular fibrillation in a young man7 and refractory idiopathic ventricular tachycardia (VT) in a newborn,8 but these reports have not documented the potential dangers associated with its use.
Although phenytoin was successful in suppressing VT in our patient, this case report highlights several important learning points. Class I AADs, including phenytoin, can increase pacing threshold through use-dependent inhibition of sodium channel function.9 Most pacemakers have algorithms that allow for potential variations in pacing thresholds.10 However, AAD-induced elevation of pacing threshold may become problematic in circumstances where the pacing threshold is already elevated, such as following ICD shock or, as in our patient, the recent use of other class I AADs. AADs can also increase the defibrillation threshold, increase VT cycle length (potentially resulting in VT undersensing) and reduce antitachycardia pacing efficacy.2 The effect of AADs on pacing threshold can be temporarily ameliorated by increasing the settings to levels that guarantee capture but may require readjustment to conserve battery life in the long term. Phenytoin exhibits zero-order pharmacokinetics, is susceptible to multiple drug interactions and has a narrow therapeutic window. Vertigo, ataxia, headache and nystagmus are common early side effects. At higher plasma concentrations, marked confusion and sedation may result. All of these changes occur acutely and are rapidly reversible. Cardiovascular side effects include bradycardia, hypotension and, occasionally, exacerbation of VT. Our patient developed side effects despite a conventional loading protocol used in treating status epilepticus. Factors that may have contributed to toxicity were the presence of severe cardiomyopathy — which may have altered tissue distribution properties and resulted in a higher availability of unbound drug — and recent lignocaine administration, although this had been ceased 6 hours earlier. Rapid loading of phenytoin for VT has been associated with fatal arrhythmogenic complications3 and should therefore be cautiously performed.
Lessons from practice
Phenytoin has class IB antiarrhythmic drug properties and is a potential treatment option for patients with refractory ventricular arrhythmia when other agents have failed or are unavailable.
However, phenytoin has a narrow therapeutic range and the potential for multiple drug interactions.
Phenytoin administration may also result in elevation of pacemaker threshold. Care should be taken with loading doses in patients with cardiomyopathy or who are pacemaker-dependent, because of the potential for loss of pacemaker capture.
- 1. Van Herendael H, Pinter A, Ahmad K, et al. Role of antiarrhythmic drugs in patients with implantable cardioverter defibrillators. Europace 2010; 12: 618-625.
- 2. Atkinson AJ Jr, Davison R. Diphenylhydantoin as an antiarrhythmic drug. Annu Rev Med 1974; 25: 99-113.
- 3. Kowey PR. Pharmacological effects of antiarrhythmic drugs. Review and update. Arch Intern Med 1998; 158: 325-332.
- 4. Leonard WA. The use of diphenylhydantoin (dilantin) sodium in the treatment of ventricular tachycardia. AMA Arch Intern Med 1958; 101: 714-717.
- 5. Conn RD. Diphenylhydantoin sodium in cardiac arrhythmias. N Engl J Med 1965; 272: 277-282.
- 6. Eddy JD, Singh SP. Treatment of cardiac arrhythmias with phenytoin. Br Med J 1969; 4: 270-273.
- 7. Golian M, Bhagirath KM, Sapp JL, et al. Idiopathic ventricular fibrillation controlled successfully with phenytoin. J Cardiovasc Electrophysiol 2011; 22: 472-474.
- 8. Sun J, Shah N, Karpawich PP, Humes R. Refractory idiopathic ventricular tachycardia in a newborn treated successfully with phenytoin: old therapies are still effective in the current era. Pediatr Cardiol 2011; 32: 76-77.
- 9. Brode SE, Schwartzman D, Callans DJ, et al. ICD–antiarrhythmic drug and ICD–pacemaker interactions. J Cardiovasc Electrophysiol 1997; 8: 830-842.
- 10. Beadle R, Williams L, Lim HS. Drug-implantable cardioverter–defibrillator interactions. Expert Rev Cardiovasc Ther 2010; 8: 1267-1273.





No relevant disclosures.