In the 2010–11 financial year, 2.4 million surgical operations were performed in Australian hospitals, most (80%) being elective non-cardiac surgery.1 Cardiac complications — myocardial infarction (MI), cardiac arrest and other serious arrhythmias, and acute heart failure — occur in about 5% of patients aged 70 years or older undergoing non-cardiac surgery.2,3 Such complications carry 30-day mortality rates between 15% and 20% and account for a third of all postoperative deaths.2,3 They also prolong hospital length of stay, increase illness burden and reduce long-term survival.4,5 Some are potentially preventable: the Australian Incident Monitoring Study found that 3.1% of adverse events in hospital resulted from inadequate or incorrect preoperative assessment or preparation of patients.6 Inadequate preoperative assessment and medical optimisation of patients also causes delays or cancellations in surgery.
In this article, we supplement evidence presented in previous guidelines7,8 relating to preoperative evaluation and management of cardiac risk in patients undergoing elective non-cardiac surgery.
The Revised Cardiac Risk Index (RCRI) is a multivariable predictive index for major perioperative cardiac complications (Box 1).9 All clinical variables contribute equally to the index (1 point each), with scores of 0, 1, 2 and ≥ 3 points corresponding to estimated risks of major cardiac complications of 0.4%, 0.9%, 7% and 11%, respectively. Low-risk patients have an RCRI score of 0, intermediate-risk patients have a score of 1 or 2, and high-risk patients have a score of 3 or more. A systematic review has shown the RCRI to discriminate well (concordance index, 0.75) between high- and low-risk patients undergoing non-cardiac surgery, but less well (concordance index, 0.64) among patients undergoing vascular surgery.10 The RCRI also does not account for age or history of hypertension; these have been included in an adapted index that better predicts cardiovascular complications in older patients.11
Functional capacity, as measured in metabolic equivalents (METs) on the basis of history or exercise testing, ranges from poor (< 4 METs) to excellent (> 10 METs). The inability to walk four blocks or climb two flights of stairs (4 METs) carries an increased perioperative cardiac risk.12
Surgically induced stress can predispose to coronary thrombosis and myocardial ischaemia. Surgical interventions can be divided into low-, intermediate- and high-risk groups, with estimated 30-day death or MI rates of < 1%, 1%–5%, and > 5%, respectively (Box 2).13 While laparoscopic surgery and regional anaesthesia confer better pain relief and earlier functional recovery than open surgery and general anaesthesia, it remains unclear whether they significantly reduce cardiac risk.14,15
An algorithm integrating the considerations discussed above in assessing cardiac fitness for surgery is outlined in Box 3,8 and a clinical case study is presented in Box 4.
Investigations should only be performed if: a) the results are expected to accurately and significantly change clinical estimates of risk; b) these altered risk estimates consistently lead to changed management decisions; and c) the resultant management changes have been shown in clinical trials to improve clinical outcomes. As situations that satisfy all three of these criteria are rare in perioperative medicine, the value of investigations, apart from a routine 12-lead electrocardiogram (ECG), is limited in preoperative cardiac management. The most useful applications may be in reclassifying intermediate-risk patients to either low-risk (surgery can safely proceed without further intervention) or high-risk (needing more detailed evaluation and use of prophylaxis), or in determining unacceptable surgical risk in high-risk patients undergoing high-risk surgery (Box 3).8
Rest echocardiography has little value in preoperative evaluation of cardiac structure and function in patients lacking clinical features of heart failure or valvular heart disease because of its inability to accurately predict perioperative events.18 A recent population-based retrospective cohort study of 264 823 patients showed no benefit in survival or hospital length of stay from rest echocardiography performed within the 6 months before surgery.19
Treadmill stress testing, dobutamine stress echocardiography (DSE) and myocardial perfusion imaging (MPI) have limited value in predicting perioperative cardiac events and are not indicated in low- or intermediate-risk patients or those undergoing low-risk surgery.20 High-risk patients (those with an RCRI score ≥ 3) or those undergoing intermediate- or high-risk surgery may be eligible for testing if the results are likely to change management. In patients unable to exercise, DSE and MPI can detect moderate to large ischaemic burden with similar accuracy.20
This assesses functional capacity more accurately than patient self-report, and measures of total oxygen consumption and anaerobic threshold (if above certain threshold values) seem to identify individuals at very low surgical risk.21 While cardiopulmonary exercise testing may provide additional prognostic information in older patients with cardiopulmonary disease or patients undergoing major thoracic or abdominal operations, there are currently insufficient data to show its routine use alters perioperative care or outcomes compared with bedside risk stratification methods.22
Biomarkers such as high-sensitivity troponin and B-type natriuretic peptide (BNP) appear to add incremental prognostic information to the RCRI.23,24 However, until adequately powered trials show such revised risk estimates change management and improve patient outcomes, biomarker tests should not be used routinely.
β-Blockers are potentially useful in lowering cardiac risk by antagonising the effects of adrenaline and other stress hormones and exerting negative chronotropic and inotropic actions. However, results of randomised trials and meta-analyses suggest mixed effects, with further uncertainty resulting from the recent disclosure of several potentially fraudulent or negligent Dutch trials.26 The large Perioperative Ischemic Evaluation Study (POISE) showed a 31% reduction in the risk of non-fatal MI with β-blockers, at the expense of a 34% increased risk of all-cause mortality and 89% increased risk of non-fatal stroke.27 A recent meta-analysis of nine well conducted “secure” trials (including POISE, and excluding the “non-secure” Dutch trials) found initiation of β-blockers before surgery caused a 27% increase in 30-day all-cause mortality and a 73% increase in non-fatal stroke, while decreasing risk of non-fatal MI by 27%.28 The updated 2009 American College of Cardiology and American Heart Association guidelines give a Class 1 recommendation only for continuing β-blockers in patients with a pre-existing cardiac condition for which there is a strong indication.29
However, two large retrospective observational studies using propensity-based risk adjustment suggest that β-blockers reduce all-cause inhospital deaths proportionally to increasing cardiac risk, as measured by an RCRI score ≥ 2, while increasing deaths in those with an RCRI score < 2 (Box 5).30,31 In the former patients, one of the studies showed that, while β-blockers reduced risk of non-fatal MI and cardiac arrest, stratified analyses indicated these benefits were limited to patients undergoing non-vascular surgery.31
It thus remains unclear which patients benefit from β-blockers. If β-blockers are to be initiated, observational data suggest they be restricted to high-cardiac-risk patients,30,31 and should be commenced some weeks before surgery and haemodynamically titrated to a tolerable dose that lowers resting heart rate to 70 beats/min.32 Longer-acting agents such as atenolol appear to be safer than short-acting agents such as metoprolol.33
Statins improve endothelial function, reduce vascular inflammation and stabilise atherosclerotic plaque. Evidence of benefit of perioperative treatment in statin-naive patients is of limited quality and is dominated by observational studies34 or trials in cardiac surgery.35 The few secure randomised trials involving patients undergoing non-cardiac vascular surgery are underpowered and inconclusive.36 Patients already prescribed statins as chronic therapy should continue treatment in the perioperative period.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have not been shown to improve outcomes in the absence of left ventricular systolic dysfunction. Indeed, observational studies suggest they predispose to severe intraoperative hypotension (generally responsive to fluid loading and vasopressors), especially if combined with β-blockers or diuretics, and may increase 30-day mortality in patients undergoing major vascular surgery.37 There is debate about whether these agents should be withheld one half-life before anaesthesia induction if their indication is purely for hypertension (unless blood pressure is uncontrolled) or, given the preponderance of day-of-surgery admissions, to recommend continuation with adequate hydration.38 A prospective randomised trial is required to clarify the safety of perioperative use of these agents.
Aspirin interacts with the cyclo-oxygenase enzyme system and irreversibly inhibits platelet aggregation, theoretically lessening risk of coronary thrombosis but increasing risk of perioperative bleeding. No adequately powered trial has assessed benefits of aspirin prophylaxis in aspirin-naive patients. In patients with known CAD, excluding those with recent coronary artery stent insertion (discussed below), risk of subsequent death or MI is increased two- to threefold if aspirin is ceased before surgery.39 While the risk of major postoperative bleeding may offset this cardiac risk for certain procedures, such as extensive skin grafting, a recent meta-analysis of 41 studies involving 49 590 surgical patients shows that, overall, the cardiac risk exceeds bleeding risk for most surgical patients with known CAD whose aspirin is withheld.40
Percutaneous coronary intervention (PCI) or coronary artery bypass grafting is only indicated before non-cardiac surgery in clinically unstable patients (those with unstable angina, recent MI or ventricular arrhythmias) with significant left main or three-vessel (or two-vessel if this includes the proximal left anterior descending artery) CAD. A large trial failed to show any perioperative or long-term benefit of prophylactic revascularisation, compared with optimal medical treatment alone, in stable patients undergoing high-risk surgery.41
Large observational studies show that symptomatic congestive heart failure (CHF) increases the absolute risk of perioperative death to 8% — more than twice the risk seen in established CAD without CHF.42 Other studies suggest stable, well controlled CHF does not necessarily increase risk.43 Current guidelines are uncertain about when left ventricular function should be reassessed using echocardiography in clinically stable patients with known CHF.7,18 The ability of BNP and N-terminal proBNP to discriminate cardiac risk among patients with CHF, who may have chronically elevated levels, has yet to be examined.18 It is also unknown whether optimising CHF management before surgery — including using a BNP-guided strategy to titrate therapy, correcting coexisting anaemia and strictly controlling ventricular rate in patients with atrial fibrillation — improves postoperative outcomes.44 What is agreed is the need to defer surgery in patients with decompensated or severe chronic CHF (worsening or new-onset CHF; New York Heart Association Class IV symptoms) until they are medically optimised and euvolaemic. In patients with newly diagnosed CHF, elective surgery should be delayed 3 months or more to allow adequate time for antifailure therapies to improve left ventricular function and remodelling.44 β-Blockers with proven mortality benefit (bisoprolol, carvedilol or metoprolol succinate) and ACE inhibitors or ARBs should be continued during the perioperative period unless precluded by hypotension or symptomatic bradycardia.
In all patients with clinical features consistent with severe valvular heart disease, preoperative echocardiography and a 12-lead ECG are mandatory in assessing valve and left ventricular dysfunction. These are also important to screen for conduction system defects caused by perivalvular fibrosis that may predispose to bradyarrhythmias requiring perioperative pacing. Ideally, patients eligible for valve reconstruction or replacement, or transcutaneous valvuloplasty or valve implantation, should undergo these procedures before elective surgery. This is particularly pertinent in patients with symptomatic or critical calcific aortic stenosis with a valve area < 0.8 cm2, which carries a 10%–28% risk of perioperative sudden cardiac death.45 Patients with severe mitral regurgitation should be medically optimised before surgery, including rate control of chronic atrial fibrillation. ACE inhibitors or ARBs prescribed for afterload reduction should be continued despite a risk of anaesthesia-induced hypotension. In the absence of prior history of infective endocarditis, mechanical prosthetic heart valves, congenital heart defects, cardiac transplantation with valvulopathy, or rheumatic heart disease in Indigenous Australians, prophylactic antibiotics are usually not required. Exceptions to this are operations associated with a high risk of bacteraemia (dental procedures and periodontal disease; genitourinary procedures; surgery involving the oropharynx, respiratory tract, sinuses, nose or ear; incision and drainage of local abscesses; or surgery through infected skin).8,46
Australian guidelines from 2009 recommend that elective surgery requiring cessation of dual antiplatelet therapy should be postponed for at least 6 weeks after insertion of bare-metal stents and 12 months after insertion of drug-eluting stents (DES).47 However, more recent American guidelines reflecting additional new evidence and experience with later-generation DES suggest a minimum period of 6 months after insertion of DES.48 A recent retrospective cohort study of more than 28 000 patients who underwent non-cardiac surgery within 2 years after stent insertion showed that major adverse cardiac events at 30 days were associated with emergency surgery, history of MI in the 6 months before surgery and an RCRI score greater than 2, but not with stent type or timing of surgery beyond 6 months after stent insertion.49 Ceasing dual therapy earlier than stipulated above carries a very high risk of stent thrombosis, with mortality rates up to 20%.50 Continuation of dual therapy confers little risk of major bleeding in most minor surgery (Box 6). In patients requiring urgent surgery associated with high bleeding risk within the recommended minimum time frames, aspirin should be continued and clopidogrel (or prasugrel or ticagrelor) withdrawn at least 5 to 7 days before surgery, depending on the agent.8,47 This should be coupled with consideration of bridging anticoagulation (heparin–tirofiban or heparin–eptifibatide) in selected highest-risk patients (although there are limited data in support of such treatments).47 Any discussion about postponing surgery or continuing, modifying or discontinuing antiplatelet therapy must involve close liaison between a patient’s GP, interventional cardiologist, anaesthetist, surgeon and haematologist to balance the risk and benefit of such decisions. Patients scheduled for PCI and requiring non-cardiac surgery in the foreseeable future should preferably receive bare-metal stents.
Whether and when to withhold anticoagulants depends on the balance between risk of thromboembolic events if interrupted and risk of major bleeding if continued (Box 6). Patients at low thromboembolic risk can cease taking anticoagulants with no need for bridging heparin, while those undergoing minor procedures with low bleeding risk do not require their cessation.51 In high-risk patients, bridging heparin is required after oral anticoagulants are ceased 5 days (for warfarin)51 or between 24 hours and 4 days (for the newer oral agents dabigatran, rivaroxaban and apixaban, as per manufacturer’s product information for each) before surgery. Bridging anticoagulation with subcutaneous low-molecular-weight heparin, if there are no contraindications, obviates the need for hospitalisation to administer intravenous unfractionated heparin. Bleeding risk with the newer anticoagulant agents is of concern, given the lack of both an antidote and reliable assays of anticoagulation effects. Early, effective and ongoing communication between GPs and specialists, combined with reference to detailed, up-to-date protocols, is required to maximise patient safety during perioperative transitions of anticoagulation.52
Obstructive sleep apnoea (OSA) affects up to 25% of adult general surgical patients and up to 77% of those undergoing bariatric surgery.53 As many as 70% of cases are undiagnosed before patients present for preoperative evaluation. In a recent meta-analysis of case–controlled and cohort studies of patients diagnosed with OSA and undergoing elective surgery, postoperative cardiorespiratory events were twice those seen in patients without OSA (3.8% v 1.7%).53 Various screening questionnaires with equivalent predictive value in identifying patients with moderate to severe OSA are easy to administer.54 In cases where known OSA is mild or screening risk is low, surgery is low risk and there are no associated comorbidities, surgery can proceed without further intervention. In all other cases, formal evaluation by a sleep physician, initiation or titration of continuous positive airway pressure (CPAP) therapy where indicated, and close liaison with anaesthetists should be undertaken. The optimal duration of CPAP therapy in newly diagnosed patients awaiting surgery and how patients with known OSA who are non-compliant with CPAP therapy should be treated remain uncertain.
Chronic obstructive pulmonary disease (COPD) frequently coexists in patients with CAD or CHF who are, or have been, smokers. COPD is an independent risk factor for major cardiopulmonary complications and can complicate assessment of functional capacity and administration of prophylactic β-blockers. Clinical history and simple bedside spirometry are sufficient to gauge disease severity in otherwise stable patients. Routine chest x-rays and formal lung function tests add little value. In the absence of moderate to severe bronchospasm, a meta-analysis supports the safety of cardioselective β-blockers in most patients with stable COPD.55 Patients with combined bronchospastic disease and CAD who are undergoing high-risk surgery might derive cardioprotective benefit from α-2 adrenergic agonists (such as clonidine).56 Before surgery, patients with unstable COPD or asthma should receive oral steroids, which do not compromise wound healing, and all patients with COPD should totally abstain from smoking for at least 6 weeks.
For patients with these devices, especially implantable cardioverter defibrillators (ICDs), GPs or anaesthetists should ideally contact the relevant cardiologist to ascertain the type of device, its indications, current settings and mode of magnetic inactivation (if applicable). Such information allows appropriate safeguards to be organised, if required, before surgery.57 Surgical diathermy, particularly in chest, head or neck surgery, can cause electrical interference that may inhibit pacemakers or trigger shocks from ICDs.
1 Revised Cardiac Risk Index9
High-risk type of surgery (see Box 2)
Ischaemic heart disease (any of: history of myocardial infarction, history of a positive exercise test, current complaint of chest pain considered to be secondary to myocardial ischaemia, use of nitrate therapy, or electrocardiogram with pathological Q waves)
History of congestive heart failure
History of cerebrovascular disease
Preoperative treatment with insulin
Preoperative serum creatinine level > 177 μmol/L
2 Estimated cardiac risk* of types of surgery13
* Risk of death or myocardial infarction within 30 days of surgery. |
|||||||||||||||
3 Algorithm for evaluating cardiac risk before non-cardiac surgery*
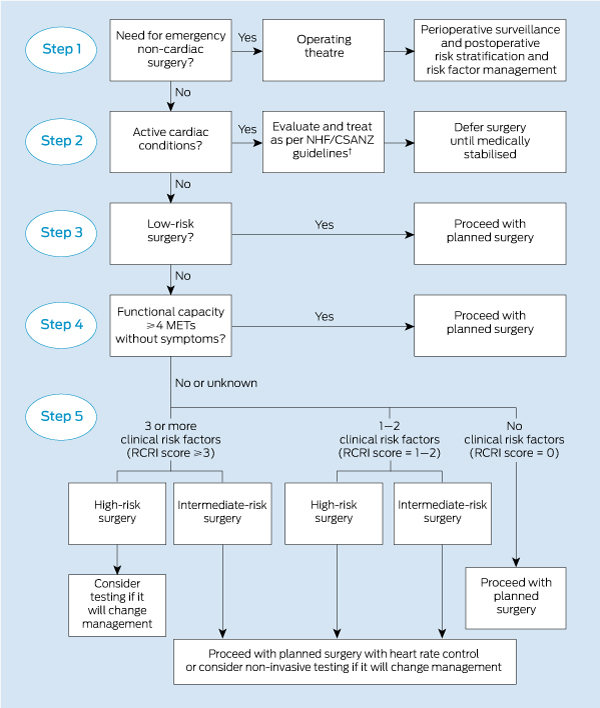
NHF/CSANZ = National Heart Foundation and Cardiac Society of Australia and New Zealand. RCRI = Revised Cardiac Risk Index. METs = metabolic equivalents. * Adapted with permission from the American College of Cardiology/American Heart Association ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery.7 † The 2006 guidelines for acute coronary syndromes16 and the 2011 update to the guidelines for heart failure.17 ◆
4 Clinical case study
5 Relation of absolute cardiac risk to β-blocker-associated reduction in all-cause inhospital death in two large observational studies*
6 Suggested risk stratification for perioperative thromboembolism and bleeding*
Provenance: Commissioned; externally peer reviewed.
- Ian A Scott1,2
- Hasan A Shohag1
- Peter C A Kam3
- Michael V Jelinek4
- Golam M Khadem1
- 1 Internal Medicine and Clinical Epidemiology, Princess Alexandra Hospital, Brisbane, QLD.
- 2 University of Queensland, Brisbane, QLD.
- 3 Royal Prince Alfred Hospital, Sydney, NSW.
- 4 St Vincent’s Hospital, Melbourne, VIC.
Michael Jelinek has received fees from Cardioscan for reporting ECGs and from insurance companies and law firms for medicolegal services, and transport and accommodation costs from Servier to attend the annual scientific meeting of the Cardiac Society of Australia and New Zealand.
- 1. Australian Institute of Health and Welfare. Surgery in Australian hospitals 2010–11. http://www.aihw.gov.au/hospitals/surgery-2010-11 (accessed Sep 2013).
- 2. McNicol L, Story DA, Leslie K, et al. Postoperative complications and mortality in older patients having non-cardiac surgery at three Melbourne teaching hospitals. Med J Aust 2007; 186: 447-452. <MJA full text>
- 3. Story DA, Leslie K, Myles PS, et al. Complications and mortality in older surgical patients in Australia and New Zealand (the REASON study): a multicentre, prospective, observational study. Anaesthesia 2010; 65: 1022-1030.
- 4. Browner WS, Li J, Mangano DT; The Study of Perioperative Ischaemia Research Group. In-hospital and long-term mortality in male veterans following noncardiac surgery. JAMA 1992; 268: 228-232.
- 5. Khuri SF, Henderson WG, DePalma RG, et al. Determinations of long-term survival after major surgery and the adverse effects of postoperative complications. Ann Surg 2005; 242: 326-343.
- 6. Kluger MT, Tham EJ, Coleman NA, et al. Inadequate pre-operative evaluation and preparation: a review of 197 reports from the Australian Incident Monitoring Study. Anaesthesia 2000; 55: 1173-1178.
- 7. Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007; 50: e159-e242.
- 8. Cardiovascular Expert Group. Therapeutic guidelines: cardiovascular. Version 6. Melbourne: Therapeutic Guidelines Ltd, 2012. http://www.tg.org.au/?sectionid=42 (accessed Nov 2013).
- 9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043-1049.
- 10. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the Revised Cardiac Risk Index. Ann Intern Med 2010; 152: 26-35.
- 11. Welten GM, Schouten O, van Domburg RT, et al. The influence of aging on the prognostic value of the revised cardiac risk index for postoperative cardiac complications in vascular surgery patients. Eur J Vasc Endovasc Surg 2007; 34: 632-638.
- 12. Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med 1999; 159: 2185-2192.
- 13. Eagle KA, Rihal CS, Mickel MC, et al. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. Circulation 1997; 96: 1882-1887.
- 14. Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006; (4): CD006231.
- 15. Kettner SC, Willschke H, Marhofer P. Does regional anaesthesia really improve outcome? Br J Anaesth 2011; 107 Suppl 1: i90-i95.
- 16. Acute Coronary Syndrome Guidelines Working Group. Guidelines for the management of acute coronary syndromes 2006. Med J Aust 2006; 184 (8 Suppl): S1-S32. <MJA full text>
- 17. Krum H, Jelinek MV, Stewart S, et al. 2011 Update to National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the prevention, detection and management of chronic heart failure in Australia, 2006. Med J Aust 2011; 194: 405-409. <MJA full text>
- 18. Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Coll Cardiol 2011; 57: 1126-1166.
- 19. Wijeysundera DN, Beattie WS, Karkouti K, et al. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: population based cohort study. BMJ 2011; 342: d3695.
- 20. Beattie WS, Abdelnaem E, Wijeysundera DN, Buckley DN. A meta-analytic comparison of preoperative stress echocardiography and nuclear scintigraphy imaging. Anesth Analg 2006; 102: 8-16.
- 21. Struthers R, Erasmus P, Holmes K, et al. Assessing fitness for surgery: a comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br J Anaesth 2008; 101: 774-780.
- 22. Stringer W, Casaburi R, Older P. Cardiopulmonary exercise testing: does it improve perioperative care and outcome? Curr Opin Anaesthesiol 2012; 25: 178-184.
- 23. Weber M, Luchner A, Seeberger M, et al. Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J 2013; 34: 853-862.
- 24. Rodseth RN, Lurati Buse GA, Bolliger D. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol 2011; 58: 522-529.
- 25. Ahn J-H, Park JR, Min JH, et al. Risk stratification using computed tomography coronary angiography in patients undergoing intermediate-risk noncardiac surgery. J Am Coll Cardiol 2013; 61: 661-668.
- 26. Erasmus Medical Centre Follow-up Investigation Committee. Report on the 2012 follow-up investigation of possible breaches of academic integrity. Sep 2012. http://cardiobrief.files.wordpress.com/2012/10/integrity-report-2012-10-english-translation.pdf (accessed Sep 2013).
- 27. Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; 371: 1839-1847.
- 28. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomised controlled trials of β-blockade to prevent perioperative death in non-cardiac surgery. Heart 2013; Jul 31 [Epub ahead of print]. doi: 10.1136/heartjnl-2013-304262.
- 29. Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. J Am Coll Cardiol 2009; 54: e13-e118.
- 30. Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353: 349-361.
- 31. London MJ, Hur K, Schwartz GG, Henderson WG. Association of perioperative β-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA 2013; 309: 1704-1713.
- 32. Flu WJ, van Kuijk JP, Chonchol M, et al. Timing of pre-operative beta-blocker treatment in vascular surgery patients: influence on post-operative outcome. J Am Coll Cardiol 2010; 56: 1922-1929.
- 33. Wallace AW, Au S, Cason BA. Perioperative β-blockade: atenolol is associated with reduced mortality when compared to metoprolol. Anesthesiology 2011; 114: 824-836.
- 34. Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333: 1149-1155.
- 35. Chopra V, Wesorick DH, Sussman JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systemic review and meta-analysis. Arch Surg 2012; 147: 181-189.
- 36. Sanders RD, Nicholson A, Lewis SR, et al. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; (7): CD009971.
- 37. Railton CJ, Wolpin J, Lam-McCulloch J, Belo SE. Renin-angiotensin blockade is associated with increased mortality after vascular surgery. Can J Anaesth 2010; 57: 736-744.
- 38. Auron M, Harte B, Kumar A, Michota F. Renin-angiotensin system antagonists in the perioperative setting: clinical consequences and recommendations for practice. Postgrad Med J 2011; 87: 472-481.
- 39. Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention — cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation — review and meta-analysis. J Intern Med 2005; 257: 399-414.
- 40. Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J 2006; 27: 2667-2674.
- 41. McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351: 2795-2804.
- 42. Hammill BG, Curtis LH, Bennett-Guerrero E, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology 2008; 108: 559-567.
- 43. Xu-Cai YO, Brotman DJ, Phillips CO, et al. Outcomes of patients with stable heart failure undergoing elective noncardiac surgery. Mayo Clin Proc 2008; 83: 280-288.
- 44. Upshaw J, Kiernan MS. Preoperative cardiac risk assessment for noncardiac surgery in patients with heart failure. Curr Heart Fail Rep 2013; 10: 147-156.
- 45. Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med 1986; 146: 2131-2134.
- 46. Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol 2008; 52: 676-685.
- 47. Cardiac Society of Australia and New Zealand. Guidelines for the use of antiplatelet therapy in patients with coronary stents undergoing non-cardiac surgery. Sydney: CSANZ, 2009. http://www.csanz.edu.au/Portals/0/Guidelines/Practice/Use%20of%20antiplatelet%20therapy%20in% 20patients%20with%20coronary%20stents%20undergoing%20non-cardiac%20surgery.pdf (accessed Oct 2013).
- 48. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e326S-e350S.
- 49. Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310: 1462-1472. doi: 10.1001/jama.2013.278787.
- 50. Howard-Alpe GM, de Bono J, Hudsmith L, et al. Coronary artery stents and non-cardiac surgery. Br J Anaesth 2007; 98: 560-574.
- 51. Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med 2003; 163: 901-908.
- 52. Martin MT, Kuchta AM, Nutescu EA. A clinician’s guide to perioperative bridging for patients on oral anticoagulation. J Pharm Pract 2010; 23: 303-312.
- 53. Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10: 753-758.
- 54. Kaw R, Chung F, Pasupuleti V, et al. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth 2012; 109: 897-906.
- 55. McGory ML, Maggard MA, Ko CY. A meta-analysis of perioperative beta blockade: what is the actual risk reduction? Surgery 2005; 138: 171-179.
- 56. Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med 2003; 114: 742-752.
- 57. Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management: executive summary. Heart Rhythm 2011; 8: e1-e18.





Summary
Perioperative cardiac complications are a common cause of death and major morbidity in patients undergoing non-cardiac surgery.
Preoperative evaluation and medical optimisation can improve outcomes, although the evidence base is limited.
Evidence of effectiveness is strongest for prophylactic use of β-blockers in high-risk patients and aspirin in patients with coronary artery disease.
Particular challenges arise among patients with heart failure or valvular heart disease or those receiving antithrombotic therapy for coronary artery stents or atrial fibrillation.
Close liaison between general practitioners, surgeons, anaesthetists and cardiologists is needed for optimising preoperative management and subsequent clinical outcomes in high-risk patients.