Chest pain is a confronting symptom for patients and clinicians alike. Some patients presenting with chest pain will have serious acute illness with a high short-term risk of mortality, but this will be excluded in most patients. Chest pain is one of the most common causes of attendance at hospital emergency departments (EDs) and a frequent cause of presentations to general practice.1 Missed diagnosis, with associated adverse outcomes, can occur when chest pain assessment is based on clinical features alone.2
Regardless of the clinical setting, a stepwise approach should be applied to patients with chest pain (Box 1). In the absence of trauma, the primary focus should be exclusion of four potentially fatal conditions: acute coronary syndrome (ACS; encompassing acute myocardial infarction and unstable angina), pulmonary embolism, aortic dissection and spontaneous pneumothorax. ACS is by far the most common of these. All these conditions may present without immediately obvious physical signs, but the latter three may be accurately excluded by rapid diagnostic testing (predominantly medical imaging). However, ACS is more challenging as it cannot be readily excluded with an acceptable level of accuracy on initial clinical evaluation or with a single investigation. After excluding these conditions, attention should be turned to chronic but serious conditions that may require additional evaluation, such as stable coronary artery disease or aortic stenosis. Next, non-life threatening conditions that may benefit from specific therapy (eg, herpes zoster, gastro-oesophageal reflux) should be considered. If the clinician is confident that all these causes have been excluded, the patient can be reassured that the chest pain is due to an insignificant cause.
Here, we focus on several evolving areas relating to the assessment of patients with possible cardiac chest pain, including risk stratification, cardiac biomarkers and the role of non-invasive testing for myocardial ischaemia and coronary artery disease. This article is aimed at all clinicians who assess patients with acute, undifferentiated chest pain. It is not a systematic review, but we have directed the reader towards these where appropriate.
There is a dichotomy in the assessment of patients with possible ACS. First, early and accurate identification of patients with ST-segment-elevation myocardial infarction (STEMI) enables provision of emergency reperfusion therapy, which has a major impact on outcome, while accurate identification of patients with other types of ACS (non-ST-segment elevation myocardial infarction [NSTEMI] or unstable angina) allows for early initiation of targeted treatment known to improve outcomes in these groups. Second, accurate exclusion of myocardial ischaemia in patients with chest pain is essential to minimise the morbidity and mortality associated with missed diagnoses, while avoiding unnecessary overinvestigation in those without the disease. However, assessment is complex because of the diversity of clinical presentations of ACS and the lack of a single diagnostic test for the entire spectrum of disease.
Despite recognition that clinical systems are imperfect, a high degree of safety in chest pain assessment processes is demanded. A recent large survey of emergency physicians suggests that the target rate of unexpected adverse outcomes in patients with a negative chest pain assessment should be < 1% at 30 days;3 this target is likely to be equally stringent in primary care. Achieving this level of safety in a timely and cost-effective fashion in an era of increasing demand on acute services presents challenges. This must be considered when the potential value and safety of new developments are assessed.
Most current diagnostic strategies for acute chest pain focus on the identification of ACS and are based on the premise that other obvious diagnoses have been excluded with accurate clinical assessment.
Systematic reviews of the diagnostic value of clinical features in the assessment of chest pain have largely been carried out in hospital settings,4 where the prevalence of serious disease is higher than in general practice. It is widely understood that no single clinical feature or combination of features can be used to exclude ACS with sufficient sensitivity to obviate the need for further investigation. Thus, a strategic approach based on clinical risk stratification, a period of observation, electrocardiography and serial biomarker evaluation has emerged. In all settings, a 12-lead electrocardiogram (ECG) should be performed immediately in patients presenting acutely with chest pain to exclude ST-segment-elevation.
In general practice, the aim should be to differentiate patients who require urgent hospital-based assessment for possible ACS from those with more stable symptoms who may be investigated on an outpatient basis. Limited access to investigations encourages the use of clinical judgement or clinically based decision rules to triage patients who can continue to be managed safely in primary care. Several such decision rules exist, but with limited validation for use in primary care. Despite the lower prevalence of coronary disease in patients presenting with chest pain in primary care, the same limitations found in hospital-based cohorts apply to the value of clinical assessment. A recent well conducted Swedish study concluded that the accuracy of clinical assessment of chest pain by general practitioners was high, but insufficient to safely rule out coronary artery disease.5 Clinicians in general practice should refer patients promptly to hospital for assessment when features suggesting a diagnosis of ACS are present (Box 2).
The value of further investigation in general practice of patients with an acute onset or ongoing symptoms is limited, given that a normal ECG cannot exclude a significant short-term risk of an adverse outcome, and serial biomarker testing is required to exclude myocardial infarction. Nevertheless, in all settings, the resting ECG has a critical role in identifying patients with ST-segment elevation who require emergency reperfusion therapy. Patients with suspected ACS and ongoing pain, pain within the past 12 hours that has resolved but with an abnormal ECG, or other high-risk features (Box 3) should be referred to hospital as an emergency. Given the release kinetics of troponin, a single troponin test may have value in assessing patients with a normal ECG and no high-risk features who present more than 12 hours after resolution of symptoms suggestive of ACS. In such cases, appropriate mechanisms must be in place for prompt review of results and referral to hospital where necessary. If these facilities are unavailable, patients should be referred to the ED for same-day chest pain assessment.
Demographic and cardiovascular risk factors, such as age and sex, influence population risk of disease but should not unduly influence the assessment of individual patients. In the absence of a clear alternative diagnosis, most patients will require additional investigation to exclude coronary artery disease, and the critical decision is usually not whether, but with what urgency, this should be undertaken. In some countries, rapid-access chest pain assessment clinics offering early assessment of patients (usually within 14 days) have become an integral part of strategies for chest pain assessment as an alternative to ED-based assessment.6 However, these have not been widely implemented in Australia, and all acute care facilities with an ED should have an evidence-based strategic approach to assessing patients with chest pain.
Patients should be stratified as being at low, intermediate or high risk of short-term adverse outcomes in the context of possible ACS, in line with the joint guidelines of the National Heart Foundation and Cardiac Society of Australia and New Zealand (NHF/CSANZ) stratifying patients with ACS (Box 3).6 This model has performed well in the ED setting, with 30-day risks of adverse cardiac outcome of 0, 7% and 26% in these risk strata, respectively, when the criteria were strictly applied in one cohort.7 Risk stratification models may have greater utility in the ED, where the prevalence of ACS is about 10% (compared with primary care, where rates are lower) and where facilities to further assess patients at increased risk are readily available. The main limitation of this risk stratification model is that few patients qualify as low risk when the criteria are strictly applied. Alternative approaches include the Thrombolysis in Myocardial Infarction (TIMI) score, the Global Registry of Acute Coronary Events (GRACE) score and the GRACE Freedom-from-Event score.8,9 These models, derived from higher-risk populations, were not designed to identify low-risk patients who do not require detailed assessment for exclusion of ACS. Consequently, none can be relied on to identify patients who can be safely discharged from the ED without some period of observation and additional investigation. Nevertheless, risk stratification is essential to guide the appropriate use of resources based on pretest probability of ACS.
Cardiac troponin levels have a central role in the diagnosis of acute myocardial infarction.10 After exclusion of ST-segment elevation and dynamic ST-segment electrocardiographic changes, serial biomarker testing identifies the remaining patients with acute myocardial infarction. Protocols for the use of serial troponin measurements have largely been based on release kinetics in experimental conditions and have tended to require waiting 6–8 hours (or longer) after presentation for the second test. Recent advances in high-sensitivity assays that allow a much shorter interval of 2 hours before the second test and incorporation of serial biomarker levels into overall risk stratification models (Box 4) have demonstrated safe accelerated processes with robust clinical outcome data.11,12 These approaches have yet to be incorporated into clinical guidelines, but almost certainly will be in the foreseeable future.
Troponin levels are considered abnormal when they exceed the 99th percentile of a healthy reference population using an assay with sufficient accuracy at this level (< 10% coefficient of variation). In practice, few available assays have possessed sufficient accuracy at this level.13 The recent development of high-sensitivity assays with this level of accuracy and lower levels of detection allows measurable troponin levels to be recorded in most of the healthy population. These assays offer the promise of being able to rule out acute myocardial infarction earlier than was possible with less sensitive assays, as well as further acceleration of risk stratification models, but with the probable cost of diminished specificity.14 This will require clinicians to have a better understanding of the causes of elevated troponin levels and the kinetics of troponin release at these new lower levels of detection, possibly by incorporating values expressing change or “delta” troponin.15 The use of delta troponin values has been incorporated into the 2011 addendum to the NHF/CSANZ guidelines, but the evidence for the best approach is still emerging.16 It is imperative that clinicians have a clear understanding of the characteristics of the local troponin assay used, as reference intervals are not transferable between different troponin assays.
In two groups of patients — those who present with symptoms of ACS and in whom myocardial infarction has been excluded, and those with a stable pattern of chest pain symptoms in whom angina cannot be excluded — additional testing is required to identify those who have prognostically important coronary artery disease or unstable angina. This is an area where well established diagnostic tests exist alongside more recent developments, such as computed tomography coronary angiography (CTCA). The anatomical and pathophysiological bases for these tests are not interchangeable, with some depending on the detection of abnormal coronary blood flow (myocardial perfusion scanning) or myocardial ischaemia (stress electrocardiography and stress echocardiography), while invasive angiography and CTCA demonstrate the anatomical basis of coronary artery disease. Each investigation has different limitations depending on patient factors and the need for contrast media and ionising radiation, and the availability of each may depend on access, cost and local expertise (Box 5).
Non-invasive testing for myocardial ischaemia or coronary artery disease is of most value to patients with intermediate pretest probability of an ACS. In patients with very low risk of coronary artery disease who have symptoms of non-ischaemic pain, other causes of chest pain should be actively excluded before investigations for myocardial ischaemia or coronary atheroma are considered. Similarly, it may be futile to embark on non-invasive testing (with an attendant risk of a false negative result) in a patient with typical symptoms and a very high risk of coronary artery disease. In such cases, prompt specialist referral for consideration of an early invasive strategy should be the first step.
Investigations may identify the presence or effects of coronary artery stenosis but, where this cannot be achieved, a broader aim is to further refine risk stratification to identify patients at low risk of an adverse outcome after discharge from the hospital or ED. Exercise stress electrocardiography has become largely obsolete as a means of diagnosing reversible myocardial ischaemia, due to insufficient diagnostic accuracy, but it retains a well established role in identifying patients with chest pain who can safely be discharged from the ED.17,18 Exercise stress electrocardiography may be limited by patients’ inability to exercise at an adequate level, non-specific electrocardiographic changes (particularly in the setting of an abnormal resting ECG), and false positive results, but it remains attractive by virtue of its low cost and widespread availability.
The combination of cardiac imaging with exercise or pharmacological stress testing can increase accuracy beyond electrocardiography alone (Box 6).19-21 In the United States and Europe, cardiac magnetic resonance imaging has emerged as a safe, non-ionising and more accurate alternative to nuclear perfusion scanning, but it remains predominantly a research tool in Australia.23
CTCA is the most rapidly evolving test for assessing patients with chest pain and is the most sensitive non-invasive test for identifying coronary artery disease.22 Recent studies have shown that this technique allows patients to be safely discharged from the ED.24 A CTCA-based strategy may also be faster than other strategies, particularly when these rely on hospital admission for myocardial perfusion scanning.24,25 However, this finding is of limited value in Australia, where myocardial perfusion scanning has not been the principal investigation for chest pain assessment.
It is important to recognise some limitations of CTCA. Elevated heart rate, coronary calcium and obesity all impair image quality. The use of iodinated contrast media is risky in patients with renal impairment or in those taking metformin. In the widely cited Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment (CT-STAT) trial, only 11% of the patients screened met the study’s inclusion criteria.25 Early studies suggested that CTCA should not be performed until after a second troponin measurement, as myocardial infarctions caused by moderate, rather than severe, coronary stenoses could potentially be missed.26 This emphasises that the strength of CTCA lies in excluding coronary atheroma. Furthermore, in the presence of known coronary artery disease, functional testing for ischaemia may be a more appropriate choice of investigation.27
Some centres perform CTCA with a total radiation dose of < 1 mSv, but in most centres, using general CT scanners without modern dose-reduction equipment, the total dose is likely to be significantly higher. Patients presenting to the ED, where there is an imperative to achieve a diagnostic study regardless of heart rate, may receive 10 mSv, although this is still lower than in most myocardial perfusion scans.28 There is now strong evidence that CT radiation can induce cancer.29 As CTCA could potentially be applied to more than 60% of patients presenting with chest pain in Australia, it is appropriate to remember that other tests are available and that CTCA has not yet demonstrated superiority in this setting. Nevertheless, CTCA is likely to become an increasingly important tool for ruling out significant coronary artery disease in patients with chest pain. Ongoing large clinical trials, such as the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE),30 should provide more definitive evidence in this area. Currently, Medicare regulations limit rebates to specialist referral for CTCA, and a robust system of credentialling has been introduced as a quality control measure.
Complex tests need to be appropriately incorporated into an overall strategy of risk-based chest pain assessment, integrating safe, accessible and cost-effective techniques that can accommodate the broadest range of patient presentations and comorbidities.
Chest pain is a common presenting symptom with many diagnostic challenges and pitfalls. Medicopolitical imperatives such as the National Emergency Access Target render the situation more complicated still. Both technology and the evidence base guiding the approach to the problem have developed considerably since the NHF/CSANZ first commissioned guidelines in this area in 2000. Clinicians can now benefit from a better understanding of risk stratification and enhanced diagnostic tools that make excluding avoidable short-term adverse events with a high degree of accuracy a realistic proposition. The challenge remains to implement these advances as widely as possible in an environment of constrained resources and increasing demand. This will be best achieved by an approach that integrates the technology and evidence into a comprehensive but straightforward and accessible strategy.
1 A pragmatic differential diagnosis of non-traumatic chest pain*
Life-threatening diagnoses that should not be missed:
Acute coronary syndrome
Acute myocardial infarction
Unstable angina pectoris
Acute pulmonary embolism
Aortic dissection
Spontaneous pneumothorax
Chronic conditions with an adverse prognosis that require further evaluation:
Angina pectoris due to stable coronary artery disease
Aortic stenosis
Aortic aneurysm
Lung cancer
Other acute conditions that may benefit from specific treatment:
Acute pericarditis
Pneumonia or pleurisy
Herpes zoster
Peptic ulcer disease
Gastro-oesophageal reflux
Acute cholecystitis
Other diagnoses:
Neuromusculoskeletal causes
Psychological causes
* This differential diagnosis is not intended to be exhaustive.
2 Case vignette
A 66-year-old man calls his general practitioner for advice. He has been treated for type 2 diabetes and primary prevention of cardiovascular disease for about 5 years. He calls from the airport where he is due to board an interstate flight but is concerned because he experienced 20 minutes of “burning” central chest discomfort while walking into the airport. He has had similar self-limiting symptoms with exertion for 4 weeks. During this time, he underwent upper gastrointestinal endoscopy that was unremarkable.
Comment: Even with the limited information available from a telephone call, and despite the atypical description of the chest pain, this patient exhibits several features suggestive of intermediate risk for an acute coronary syndrome.6 As such, he should be advised to attend hospital for assessment of chest pain without delay.
The patient is reviewed in the emergency room, where he has an unremarkable electrocardiogram and a cardiac troponin I level of 0.01 μg/L on admission, and 0.02 μg/L 6 hours and 25 minutes later (99th percentile of a healthy reference population, 0.04 μg/L). After the second troponin measurement, an exercise stress echocardiogram is strongly positive at a low workload, with reproduction of the index symptoms at < 50% of the predicted workload and evidence of reversible ischaemia in the territory of the left anterior descending coronary artery. He has cardiac catheterisation the same day and is found to have a critical stenosis of the mid left anterior descending artery, which is treated with percutaneous coronary intervention and deployment of a drug-eluting stent. He makes an uneventful recovery, with normal left ventricular function.
3 Features associated with high risk, intermediate risk and low risk of adverse short-term outcomes in patients presenting with chest pain due to possible acute coronary syndrome
High risk
Presentation with clinical features consistent with acute coronary syndrome (ACS) and any of the following features:
Repetitive or prolonged (> 10 minutes) ongoing chest pain or discomfort
Elevated level of at least one cardiac biomarker (troponin recommended)
Persistent or dynamic electrocardiographic changes of ST-segment depression ≥ 0.5 mm or T-wave inversion ≥ 2 mm
Transient ST-segment elevation of ≥ 0.5 mm in more than two contiguous leads
Haemodynamic compromise
Sustained ventricular tachycardia
Syncope
Significant left ventricular (LV) dysfunction (LV ejection fraction < 40%)
Prior percutaneous coronary intervention within 6 months or prior coronary artery bypass surgery
Presence of diabetes or chronic kidney disease (estimated glomerular filtration rate < 60 mL/minute) and typical symptoms of ACS
Intermediate risk
Presentation with clinical features consistent with ACS and any of the following features, without high-risk features:
Chest pain or discomfort within the past 48 hours that occurred at rest, or was repetitive or prolonged (but currently resolved)
Age > 65 years
Known coronary artery disease
Prior aspirin use
Two or more of: hypertension, family history, current smoking, hyperlipidaemia
Presence of diabetes or chronic kidney disease and atypical symptoms of ACS
Low risk
Presentation with clinical features consistent with ACS without intermediate-risk or high-risk features. This includes onset of anginal symptoms within the past month, or worsening in severity or frequency of angina, or lowering of anginal threshold.
* Adapted from Box 8 in the National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand Guidelines for the management of acute coronary syndromes 2006.6 Copyright 2006 The Medical Journal of Australia. Used with permission.
4 A proposed algorithm, incorporating an accelerated diagnostic protocol,* for assessment of possible cardiac chest pain after exclusion of ST-segment elevation on initial ECG
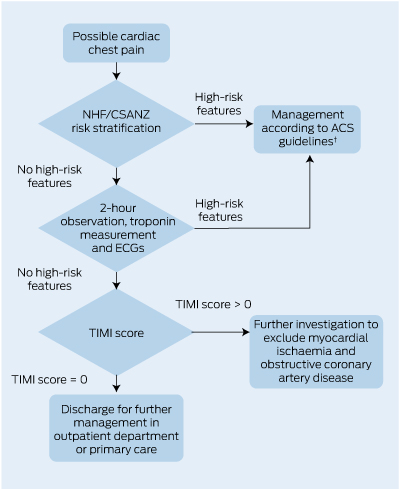 | |||||||||||||||
|
NHF/CSANZ = National Heart Foundation and Cardiac Society of Australia and New Zealand. ACS = acute coronary syndrome. ECG = electrocardiogram. TIMI = Thrombolysis in Myocardial Infarction. * Based on the ADAPT study.11 † NHF/CSANZ Guidelines for the management of acute coronary syndromes 2006.6 | |||||||||||||||
5 Features of non-invasive tests available for further risk stratification of patients with chest pain, after excluding acute myocardial infarction
|
Procedural considerations |
Relative contraindications* |
|||||||||||||
Condition and test |
Cost† |
Radiation |
Iodinated contrast media |
Inability to exercise |
Significant resting ECG abnormality‡ |
Renal impairment |
Obesity |
Severe airway disease |
|||||||
Myocardial ischaemia or perfusion |
|
|
|
|
|
|
|
|
|||||||
Exercise stress electrocardiography |
$ |
No |
No |
Yes |
Yes |
No |
Yes |
Yes§ |
|||||||
Stress echocardiography |
$$ |
No |
No |
No¶ |
Yes |
No |
Yes |
No¶ |
|||||||
Myocardial perfusion scanning |
$$$ |
Yes |
No |
No¶ |
No |
No |
Yes |
Yes§** |
|||||||
Obstructive coronary artery disease |
|
|
|
|
|
|
|
|
|||||||
Computed tomography coronary angiography |
$$ |
Yes |
Yes |
No |
No |
Yes |
Yes |
No |
|||||||
ECG = electrocardiogram. * Relative contraindications should be discussed further for individual patients. † Relative cost indications are based on current Medicare rebates. ‡ For example, left bundle branch block. § If there is significant functional impairment. ¶ If pharmacological stress testing can be performed. ** Adenosine is contraindicated. |
|||||||||||||||
6 Representative performance characteristics of non-invasive tests to identify myocardial ischaemia or obstructive coronary artery disease in patients with chest pain
Test |
Sensitivity |
Specificity |
|||||||||||||
Exercise stress electrocardiography21 |
68% |
77% |
|||||||||||||
Stress echocardiography20 |
83% |
77% |
|||||||||||||
Exercise stress myocardial perfusion scanning19 |
85%–90% |
70%–75% |
|||||||||||||
Computed tomography coronary angiography22 |
99% |
89% |
|||||||||||||
Provenance: Commissioned; externally peer reviewed.
- William A Parsonage1
- Louise Cullen2
- John F Younger3
- Royal Brisbane and Women’s Hospital, Brisbane, QLD.
William Parsonage and Louise Cullen receive research grant support from the Queensland Emergency Medicine Foundation.
William Parsonage and Louise Cullen are in receipt of research grants from Abbott Diagnostics, Roche, Siemens and Alere and have received honoraria from Abbott Diagnostics. William Parsonage has received consulting fees and travel expenses from AstraZeneca and Sanofi Aventis, consulting fees from Hospira and travel expenses from Abbott Pharmaceuticals and Abbott Diagnostics. Louise Cullen has received honoraria and travel expenses from Alere and travel expenses from Pfizer and Boehringer Ingelheim. John Younger has received payment for educational activities from Philips Medical Systems and AstraZeneca and travel expenses from Pfizer and Servier.
- 1. Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007; (386): 1-32.
- 2. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000; 342: 1163-1170.
- 3. Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the emergency department? A clinical survey. Int J Cardiol 2013; 166: 752-754.
- 4. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005; 294: 2623-2629.
- 5. Nilsson S, Ortoft K, Molstad S. The accuracy of general practitioners’ clinical assessment of chest pain patients. Eur J Gen Pract 2008; 14: 50-55.
- 6. Aroney CN, Aylward P, Kelly A-M, et al; Acute Coronary Syndrome Guidelines Working Group. Guidelines for the management of acute coronary syndromes 2006. Med J Aust 2006; 184 (8 Suppl): S1-S30. <MJA full text>
- 7. Cullen L, Parsonage WA, Greenslade J, et al. Comparison of early biomarker strategies with the Heart Foundation of Australia/Cardiac Society of Australia and New Zealand guidelines for risk stratification of emergency department patients with chest pain. Emerg Med Australas 2012; 24: 595-603.
- 8. Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284: 835-842.
- 9. Brieger D, Fox KA, Fitzgerald G, et al. Predicting freedom from clinical events in non-ST-elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 2009; 95: 888-894.
- 10. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020-2035.
- 11. Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol 2012; 59: 2091-2098.
- 12. Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet 2011; 377: 1077-1084.
- 13. Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010; 31: 2197-2204.
- 14. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858-867.
- 15. Scott IA, Cullen L, Tate JR, Parsonage W. Highly sensitive troponin assays — a two-edged sword? Med J Aust 2012; 197: 320-323. <MJA full text>
- 16. Chew DP, Aroney CN, Aylward PE, et al. 2011 Addendum to the National Heart Foundation of Australia/Cardiac Society of Australia and New Zealand Guidelines for the management of acute coronary syndromes (ACS) 2006. Heart Lung Circ 2011; 20: 487-502.
- 17. Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation 2010; 122: 1756-1776.
- 18. Aroney CN, Dunlevie HL, Bett JH. Use of an accelerated chest pain assessment protocol in patients at intermediate risk of adverse cardiac events. Med J Aust 2003; 178: 370-374. <MJA full text>
- 19. Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging 2004; 31: 261-291.
- 20. Beleslin BD, Ostojic M, Djordjevic-Dikic A, et al. Integrated evaluation of relation between coronary lesion features and stress echocardiography results: the importance of coronary lesion morphology. J Am Coll Cardiol 1999; 33: 717-726.
- 21. Detrano R, Gianrossi R, Froelicher V. The diagnostic accuracy of the exercise electrocardiogram: a meta-analysis of 22 years of research. Prog Cardiovasc Dis 1989; 32: 173-206.
- 22. Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 2008; 94: 1386-1393.
- 23. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012; 379: 453-460.
- 24. Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012; 366: 1393-1403.
- 25. Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol 2011; 58: 1414-1422.
- 26. Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol 2009; 53: 1642-1650.
- 27. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation 2010; 122: e525-555.
- 28. Nasis A, Meredith IT, Nerlekar N, et al. Acute chest pain investigation: utility of cardiac CT angiography in guiding troponin measurement. Radiology 2011; 260: 381-389.
- 29. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499-505.
- 30. Duke University. Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE). ClinicalTrials.gov identifier NCT01174550. http://clinicaltrials.gov/show/NCT01174550 (accessed Mar 2013).





Summary
Chest pain is a common reason for presentation in hospital emergency departments and general practice.
Some patients presenting with chest pain to emergency departments and, to a lesser extent, general practice will be found to have a life-threatening cause, but most will not. The challenge is to identify those who do in a safe, timely and cost-effective manner.
An acute coronary syndrome cannot be excluded on clinical grounds alone.
In patients with ongoing symptoms of chest pain, without an obvious other cause, ST-segment-elevation myocardial infarction should be excluded with a 12-lead electrocardiogram at the first available opportunity.
Significant recent advances in the clinical approach to patients with acute chest pain, including better understanding of risk stratification, increasingly sensitive cardiac biomarkers and new non-invasive tests for coronary disease, can help clinicians minimise the risk of unexpected short-term adverse cardiac events.
An approach that integrates these advances is needed to deliver the best outcomes for patients with chest pain. All hospital emergency departments should adopt such a strategic approach, and general practitioners should be aware of when and how to access these facilities.