Clinical record
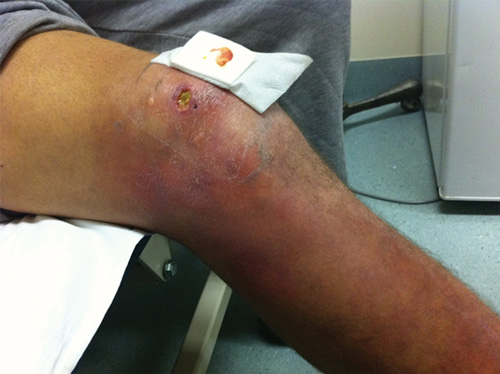
A 19-year-old man with a 3-month history of a non-healing ulcer of the right knee and indurated oedema involving the entire lower leg (Box 1) presented after not responding to multiple courses of antibiotics. A punch biopsy showed necrosis of the dermis and subcutaneous tissues, and extensive infiltration with extracellular acid-fast bacilli on auramine–rhodamine staining. Polymerase chain reaction (PCR) testing for the IS2404 insertion sequence of Mycobacterium ulcerans was positive,1 and M. ulcerans was isolated from culture at 6 weeks. The patient reported holidaying on the Bellarine Peninsula, an endemic area for M. ulcerans, 6 months before the development of the lesion. Surgical intervention was deferred due to the extensive area involved and World Health Organization guidelines suggesting antibiotics as first-line treatment for extensive oedematous disease.2
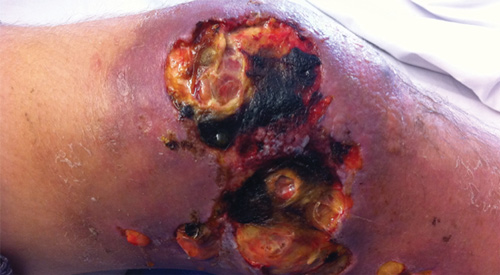
Four weeks later, there was a rapid deterioration in clinical condition, with fevers, sweats, anorexia and a recurrence of lower limb oedema and pain. Significant skin breakdown occurred around the ulcer and distally on the leg, with copious serous exudate (Box 2). Full blood examination showed a neutrophilia (neutrophil count, 14.9 × 109/L [reference interval, 1.9–8.0 × 109/L]) and a raised C-reactive protein level (69.8 mg/L [reference interval, < 3 mg/L]). Prednisolone 50 mg daily and
Mycobacterium ulcerans infection is a geographically restricted infection often referred to by its local name as Daintree, Buruli or Bairnsdale ulcer (BU). The disease occurs in sub-Saharan Africa and within discrete regions of Australia, predominantly coastal Victoria and northern Queensland. Significant diagnostic delays, which place patients at risk of more extensive disease, are common. A recent review identified a mean duration of 42 days of symptoms before diagnosis (range, 2–270 days).3 These delays often occur when patients present outside endemic areas, a factor affected by the long incubation period of 4–7 months.4
BU is characterised by slowly progressive skin lesions with local necrosis, destruction of lipocytes and surprisingly little systemic inflammation. This unusual pattern of infection is the result of its unique virulence factor mycolactone, an immunomodulatory toxin that induces necrosis of host tissues and can limit initiation of immune responses and the recruitment of inflammatory cells.5 Following the increased use of antibiotics in BU, there have been descriptions of worsening clinical condition after an initial response to therapy.6 Such deteriorations (referred to as paradoxical reactions) are most common in patients with extensive disease and can take a variety of forms including increased ulcer size, ulceration of previously non-ulcerative papules or the development of new lesions not detectable before antibiotics.7 It is particularly important that paradoxical reactions are not misinterpreted as antibiotic failure, as the vast majority of lesions will resolve without a change in antibiotic regimen.
Paradoxical reactions may be the result of antibiotic-induced suppression of mycolactone synthesis, leading to decreased mycolactone concentrations and thus a reversal of macrophage and neutrophil dysfunction with renewed immune surveillance and response.7 There are many parallels to the paradoxical reactions described with Mycobacterium tuberculosis in HIV co-infected patients who initiate antiretroviral therapy. In that setting, the reaction likely represents an increased inflammatory response secondary to antiretroviral-induced immune reconstitution. As with M. tuberculosis, paradoxical reactions to BU commonly occur 4–8 weeks after therapy commences.7
It has been shown in animal models that corticosteroids do not adversely affect the outcome of antibiotic therapy in BU,8 and Friedman and colleagues reported a marked improvement in the appearance of lesions when steroids were used for paradoxical reactions in this setting.9 Established dosing guidelines are available for the management of paradoxical reactions to M. tuberculosis (most popularly, 1 mg/kg/day until improvement, followed by a 2-week wean),10 yet the degree of applicability of these guidelines to BU management is unknown.
Lessons from practice
Mycobacterium ulcerans should be considered in patients who present with non-healing chronic ulcers or atypical cellulitis that do not respond to standard treatment.
It is common for M. ulcerans infections to worsen following the initiation of antibiotics; these paradoxical reactions are likely due to an enhanced immune response directed at dead and dying bacteria and do not reflect a failure of antibiotic therapy.
Antibiotics should be continued unchanged when a paradoxical reaction occurs.
Steroids may play a role in diminishing skin loss and systemic symptoms associated with paradoxical reactions.
- 1. Ross BC, Marino L, Oppedisano F, et al. Development of a PCR assay for r apid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol 1997; 35: 1696-1700.
- 2. World Health Organization. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer). http://www.who.int/buruli/information/antibiotics/en/index.html (accessed Mar 2012).
- 3. Boyd SC, Athan E, Friedman ND, et al. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med J Aust 2012; 196: 341-344. <MJA full text>
- 4. Lavender CJ, Fyfe JA, Azuolas J, et al. Risk of Buruli ulcer and detection of Mycobacterium ulcerans in mosquitoes in southeastern Australia. PLoS Negl Trop Dis 2011; 5: e1305.
- 5. Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol 2005; 7: 1295-1304.
- 6. O’Brien DP, Robson ME, Callan PP, McDonald AH. “Paradoxical” immune-mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med J Aust 2009; 191: 564-566. <MJA full text>
- 7. Nienhuis WA, Stienstra Y, Abass KM, et al. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin Infect Dis 2012; 54: 519-526.
- 8. Martins TG, Trigo G, Fraga AG, et al. Corticosteroid-induced immunosuppression ultimately does not compromise the efficacy of antibiotherapy in murine Mycobacterium ulcerans infection. PLoS Negl Trop Dis 2012; 6: e1925.
- 9. Friedman ND, McDonald AH, Robson ME, O’Brien DP. Corticosteroid use for paradoxical reactions during antibiotic treatment for Mycobacterium ulcerans. PLoS Negl Trop Dis 2012; 6: e1767.
- 10. American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep 2003; 52 (RR-11): 1-77.





We thank the clinical photography unit at the Austin Hospital and the laboratory staff who performed PCR testing.
No relevant disclosures.