We present the first clinical descriptions of immune-mediated paradoxical reactions to effective antibiotic treatment for Mycobacterium ulcerans infection, which result in clinical deterioration after initial improvement. Recognition of this phenomenon could prevent unnecessary changes to antibiotic regimens, and might obviate the need for, or reduce the extent of, further surgery. (MJA 2009; 191: 564-566)
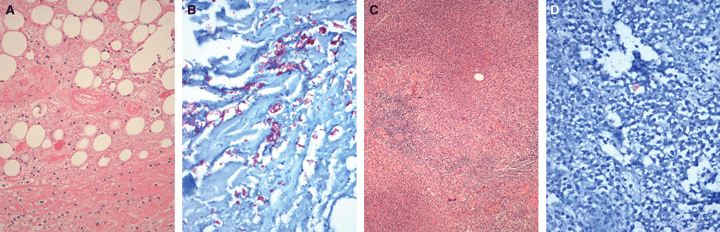
A 46-year-old man presented with a 3-month history of a slowly enlarging asymptomatic lesion on the lateral aspect of his right leg, that ulcerated and reached a size of 1.5 cm in diameter. A diagnosis of Mycobacterium ulcerans infection was confirmed by histopathological examination, which showed the classic appearance of a necrotic lesion involving the dermis and subcutaneous tissue, with a sparse acute inflammatory reaction, fat cell ghosts and large numbers of extracellular acid-fast bacilli (AFB)1 (Box, A and Box, B). In addition, culture and polymerase chain reaction (PCR) tests of excised tissue were positive for M. ulcerans.
Histopathological examination of the excised tissue showed extensive undermining necrosis in the fat and subcutaneous tissue, accompanied by a florid mixed inflammatory reaction with multinucleated giant cells (Box, C). However, only a few sparse AFB were seen (Box, D). Mycobacterial cultures of the aspirate and the excised tissue were negative at two separate laboratories. The patient completed a further 7 weeks of postoperative antibiotic therapy and, after 6 months, there was no evidence of further M. ulcerans lesions.
A “paradoxical” reaction describes a deteriorating response to treatment of an infection after initial improvement.2 Such reactions have frequently been described in infections with a number of mycobacterial species, including those causing tuberculosis, avium-intracellulare complex and leprosy, and although they occur most commonly in severely immunosuppressed patients with HIV/AIDS who are undergoing antiretroviral therapy, they can occur in immunocompetent hosts.2,3 The pathogenesis relates to an enhanced immune response to mycobacterial antigens on treatment that produces deleterious clinical effects.1 Its immune basis means that this phenomenon is also referred to as “immune reconstitution syndrome”.
M. ulcerans causes necrotising lesions of skin and subcutaneous tissue. Despite large numbers of extracellular mycobacteria, lesions are characterised by a poor inflammatory response.4 This is probably induced by immuno-inhibitory characteristics of mycolactone, the exotoxin produced by M. ulcerans,5,6 which plays a crucial role in the pathogenesis of infection with this organism. Treatment primarily involves surgical excision; however, recent evidence has shown that antibiotics can have an effective role,7,8 and their use is now recommended.9,10
Clinical paradoxical reactions during the treatment of M. ulcerans have not been previously described. Clinical deterioration during antibiotic treatment can be interpreted as treatment failure, leading to further expensive and potentially disfiguring surgery, and a change in antibiotic regimens or a prolongation of their use. In the two cases we describe, involving treatment of M. ulcerans infections in patients from an endemic area in the Bellarine Peninsula of south-eastern Australia,7 initial improvement during antibiotic therapy was followed by a paradoxical worsening in the clinical appearance. This was first interpreted as treatment failure, but we believe it was subsequently shown to result from an immune-mediated reaction to effective antibiotic treatment.
There is published histological evidence to support M. ulcerans-associated paradoxical reactions in patients being treated with antibiotics. Antibiotic therapy for M. ulcerans has been shown to lead to an apparent reversal of the immune-tolerant state of active M. ulcerans infection, with phagocytosis of mycobacteria and a rapid onset of local cellular immune responses.1 These responses are characterised by the formation of epithelioid granuloma with multinucleated giant cells and large clusters of lymphocytes. It is likely that antibiotics facilitate this immune reaction by reducing the production of the immuno-inhibitory exotoxin mycolactone,1 and also by liberating mycobacterial antigens from dead organisms.
The evidence for a paradoxical reaction in our cases included: (i) persisting M. ulcerans organisms in affected tissue after the initial surgery; (ii) an initial clinical improvement in the M. ulcerans lesions during antibiotic therapy, followed by significant clinical deterioration 1–2 months after antibiotic therapy was commenced; (iii) negative cultures from aspirates and excised tissue, suggesting that remaining mycobacteria were not alive as would be expected in a lesion that was worsening as a result of uncontrolled infection; (iv) the lack of mycobacteria seen on microscopy of the excised tissue, compared with active lesions that usually show large numbers of extracellular mycobacteria;4 and (v) significant inflammatory reactions evident on histopathological examination of excised tissue, in contrast to what was observed in the initial active lesions, and consistent with recent descriptions of immune responses in successfully treated M. ulcerans infections.1 Thus, we propose that rather than progression of the lesions because of failure of antibiotic treatment, these cases represent an adverse consequence of effective antibiotic treatment.
By applying lessons learnt from the management of immune-mediated reactions to infections with Mycobacterium tuberculosis,2 the recognition of paradoxical reactions during treatment of M. ulcerans infections might significantly influence the management of these infections. First, rather than ceasing the antibiotic therapy or changing the regimen, we advocate that therapy be continued, and that it not be changed unless there is evidence of antibiotic failure on histopathological examination or culture. Further, prolongation of the intended duration of antibiotic therapy is probably not required. Second, it may be possible that repeated needle aspiration for fluctuant lesions, as we described in Patient 1, may be effective in settling the lesion. This has been shown for lesions caused by M. tuberculosis,11 and would avoid further surgery, which, in Patient 1, included costly and disfiguring skin grafts. Finally, adjunctive corticosteroid therapy may help to settle the lesion and obviate the need for, or reduce the extent of, surgical intervention.12
- 1. Schutte D, Umboock A, Pluschke G. Phagocytosis of Mycobacterium ulcerans in the course of rifampicin and streptomycin chemotherapy in Buruli ulcer lesions. Br J Dermatol 2009; 160: 273-283. <PubMed>
- 2. Lipman M, Breen R. Immune reconstitution inflammatory syndrome in HIV. Curr Opin Infect Dis 2006; 19: 20-25.
- 3. Carvalho AC, De Iaco G, Saleri N, et al. Paradoxical reaction during tuberculosis treatment in HIV-seronegative patients. Clin Infect Dis 2006; 42: 893-895.
- 4. Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis 2003; 9: 651-656.
- 5. Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol 2005; 7: 1295-1304.
- 6. Coutanceau E, Decalf J, Martino A, et al. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J Exp Med 2007; 204: 1395-1403.
- 7. O’Brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australian case series. Med J Aust 2007; 186: 58-61. <MJA full text>
- 8. Chauty A, Ardant MF, Adeye A, et al. Promising clinical efficacy of streptomycin–rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob Agents Chemother 2007; 51: 4029-4035.
- 9. World Health Organization. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease. Geneva: WHO, 2004.
- 10. Johnson PD, Hayman JA, Quek TY, et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med J Aust 2007; 186: 64-68. <MJA full text>
- 11. Hawkey CR, Yap T, Pereira J, et al. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis 2005; 40: 1368-1371.
- 12. Garcia Vidal C, Rodríguez Fernández S, Martínez Lacasa J, et al. Paradoxical response to antituberculous therapy in infliximab-treated patients with disseminated tuberculosis. Clin Infect Dis 2005; 40: 756-759.





None identified.