Stage at diagnosis is critical in determining the probability of survival with colorectal cancer (CRC). In randomised controlled trials, population screening using faecal occult blood tests (FOBT) results in earlier stage at diagnosis for screen-detected cancers,1 and reduced mortality from colorectal malignancy compared with controls.2-4 Evaluations of cancer prevention programs with mortality as an end point take many years to complete. However, we know that early stage at diagnosis is linked to better prognosis and reduced mortality from CRC, so stage at diagnosis can serve as a surrogate marker for population mortality, and provides an early signal of program benefit.
CRC stage was defined according to the Australian Clinico-Pathological Staging System (ACPS), with stages graded from A to D in order of increasing disease spread.5 Experienced SACR staff extracted ACPS stage from clinical reports. Where stage data were incomplete, additional information was sought from three public hospital-based cancer registries.
We identified 3481 eligible patients with CRC reported to the SACR. Of these, 221 were allocated to the invited cohort. Staging data were available for 87.0% of patients: no data were available for 6.6%, and a further 6.4% had insufficient data to determine ACPS stage. The invited cohort differed significantly from all other patients in age, SES and remoteness (Box 1).
The stage profiles of the invited cohort compared to the rest of the study population (where stage was known) are shown in Box 2. The difference in stage profiles was highly significant (χ2 = 39.5; P < 0.001; Box 3). In the invited group, the percentage of stage A cancers was 34.8%, versus 19.2% in all other patients (P < 0.001). Similarly, the percentage of stage D cancers was 5.4% in the invited group versus 12.4% in all other patients (P = 0.002).
Analyses that included or excluded patients with unknown cancer stage had no effect on the statistical significance of any of the findings. Multivariate analyses showed that age and SES were significantly associated with stage at diagnosis (Box 4). However, stage A lesions were significantly more likely to be diagnosed than stage B, C or D CRC in the invited cohort relative to all other patients, while controlling for age, SES and remoteness. Stage A lesions were also more likely to be diagnosed in the participant and positive subgroups.
Earlier detection of CRC has a major impact on survival. United Kingdom data show 5-year relative survival rates of > 90% for Dukes’ stage A cancer and < 7% for Dukes’ stage D (Dukes’ cancer stages are graded A–D in order of increasing spread and metastases).6 As randomised controlled trials have shown that CRCs detected through screening are diagnosed at an earlier stage, and screened populations had reduced mortality relative to control populations,1-4 it is valid to use downstaging as a surrogate for effect on mortality. The significantly earlier stage profile in patients who participated in the NBCSP should lead to reduced mortality rates in this population. Although at the moment, only a relatively small proportion of the eligible Australian population is offered screening each year, the proposed gradual expansion of the NBCSP should result in greater reductions in CRC mortality over time, assuming that participation rates remain stable or increase.
Our findings are consistent with an earlier report using a hospital-based database of CRC patients, which showed an earlier stage distribution in people self-reporting that they were diagnosed through the NBCSP, compared with stage in symptomatic patients (ACPS stage I, 40% in those diagnosed through the NBCSP versus 14% in non-participants; and stage IV, 3% in those diagnosed through the NBCSP versus 15% in non-participants).7 However, that study did not assess all CRCs diagnosed in the entire population. Further, the study was subject to recall bias and did not analyse results on an intention-to-screen basis. Our study included all cases of CRC reported to the SACR over the periods of implementation of the NBCSP pilot program and Phase I trial, and was based on an intention-to-screen analysis, which has allowed us to avoid sampling, temporal and follow-up quality bias.
Our results are also consistent with overseas evaluations of national CRC screening programs, although the methods used vary depending on the health system. The National Bowel Cancer Screening Programme in England reported a shift towards earlier stage disease in participants compared with patients with cancer diagnosed before the screening program.8 However, it is difficult to determine whether that downstaging represents improvement in practice over time or whether it was a direct result of the program. A decrease in the proportion of more advanced stage tumours for both men and women (but significant only in men) was also seen in the early stages of the English bowel cancer screening program, in a comparison of those who took up the offer of screening with those who did not,9 but the effect was not compared with stage distribution in patients diagnosed outside of the program. The Scottish CRC screening demonstration pilot study found a high proportion of cancers at Dukes’ stage A (almost 50%) when screening with guaiac faecal occult blood testing (gFOBT).10 A similar high proportion of stage A cancers was observed in the French pilot study.11 Unlike the overseas programs, Australia’s NBCSP uses the FIT, and this is the first report of downstaging in a mass screening program using this testing method.
Although this is an observational study and it could be argued that other factors might have influenced stage, it was possible to adjust for a number of potential confounders. A second concern was that it was impossible to directly attribute an invitation to the NBCSP to a specific diagnosis of CRC. However, allocating patients to the invited cohort on the basis of a diagnosis between 14 and 366 days from the date of invitation is reasonable, considering the time taken for the clinical steps to final diagnosis after a positive test result; 14 days would appear to be the shortest time to a diagnosis. This timeline from the date of referral for colonoscopy to a diagnosis of CRC is consistent with results of studies across different health systems.12
1 Demographics of patients invited to the National Bowel Cancer Screening Program compared with those of all other patients in the study population
Remoteness index§ (based on Accessibility/Remoteness Index of Australia) |
|||||||||||||||
2 Distribution of colorectal cancer stage in patients (where stage was known; n = 3026) according to whether they were invited to participate in the National Bowel Cancer Screening Program (NBCSP)*
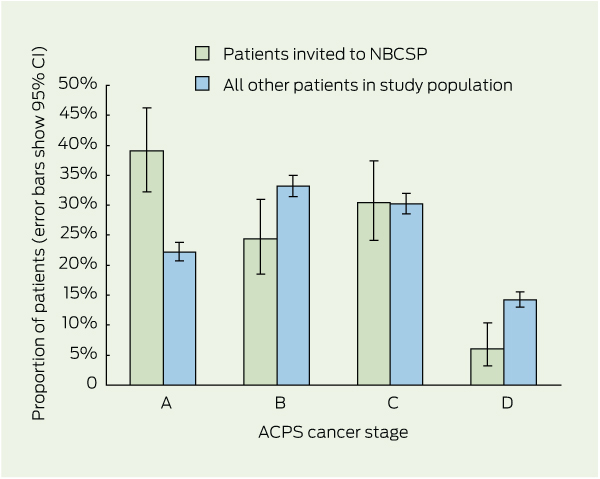
3 Colorectal cancer stage distribution of patients who were invited to, participated in or had positive test results in the National Bowel Cancer Screening Program (NBCSP), compared with the study population excluding the group of interest
Patients invited to NBCSP versus all other patients* |
Patients who participated in NBCSP versus all other patients† |
Patients with positive test results in NBCSP versus all other patients‡ |
|||||||||||||
Received 5 September 2012, accepted 17 January 2013
- Stephen R Cole1,2
- Graeme R Tucker3
- Joanne M Osborne2
- Susan E Byrne2
- Peter A Bampton2
- Robert J L Fraser2
- Graeme P Young2
- 1 Bowel Health Service, Repatriation General Hospital, Adelaide, SA.
- 2 Flinders Centre for Innovation in Cancer, Flinders University, Adelaide, SA.
- 3 Department of Health and Ageing, Adelaide, SA.
This study was funded by the Department of Health and Ageing. The Department had no control or influence over the content of this report.
No relevant disclosures.
- 1. Hewitson P, Glasziou P, Irwig L, et al. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007; (1): CD001216.
- 2. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328: 1365-1371.
- 3. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348: 1467-1471.
- 4. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1472-1477.
- 5. Davis NC, Newland RC. Terminology and classification of colorectal adenocarcinoma: the Australian clinico-pathological staging system. Aust N Z J Surg 1983; 53: 211-221.
- 6. National Cancer Intelligence Network. Colorectal cancer survival by stage – NCIN data debriefing. June 2009. http://www.ncin.org.uk/publications/data_briefings/colorectal_cancer_survival_by_stage.aspx (accessed Nov 2009).
- 7. Ananda SS, McLaughlin SJ, Chen F, et al. Initial impact of Australia’s National Bowel Cancer Screening Program. Med J Aust 2009; 191: 378-381. <MJA full text>
- 8. Ellul P, Fogden E, Simpson CL, et al. Down-staging of colorectal cancer by the National Bowel Cancer Screening Programme in England: first round data from the first centre. Colorectal Dis 2010; 12: 420-422.
- 9. Taylor EF, Morris EJA, Thomas JD, et al. Major improvement in the stage profile of tumours diagnosed in the NHS Bowel Cancer Screening Programme. Gut 2010; 59: A31. doi: 10.1136/gut.2009.208975v.
- 10. Steele RJ, McClements PL, Libby G, et al. Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009; 58: 530-535.
- 11. Goulard H, Boussac-Zarebska M, Ancelle-Park R, Bloch J. French colorectal cancer screening pilot programme: results of the first round. J Med Screen 2008; 15: 143-148.
- 12. Thompson MR, Heath I, Swarbrick ET, et al. Earlier diagnosis and treatment of symptomatic bowel cancer: can it be achieved and how much will it improve survival? Colorectal Dis 2011; 13: 6-16.





Abstract
Objective: To assess the impact of the National Bowel Cancer Screening Program (NBCSP) in South Australia.
Design, setting and participants: A cohort comparison of colorectal cancer (CRC) patient data from the NBCSP register and the South Australian Cancer Registry. Patient records of those invited to take part in screening through the NBCSP, those who participated in the program, and those with positive test results were compared with those of the rest of the study population (excluding the group of interest) on an intention-to-screen basis.
Main outcome measure: Stage of CRC at diagnosis as a surrogate marker for effect on CRC mortality.
Results: Of 3481 eligible patients, 221 had been invited to the NBCSP. Invitees were more likely to have stage A lesions compared with all other patients (34.8% versus 19.2%; P < 0.001), and half as likely to have stage D CRC (5.4% versus 12.4%; P < 0.001). A further shift towards earlier stage was seen in those who participated in screening and those with positive test results compared with all other patients (38.8% stage A and 3.0% stage D in screening participants versus 19.3% stage A and 12.4% stage D in all other patients; and 39.7% stage A and 2.6% stage D in those with positive test results versus 19.3% stage A and 12.4% stage D in all other patients; P < 0.001).
Conclusions: CRCs were diagnosed at a significantly earlier stage in people invited to the NBCSP compared with those who were not invited, regardless of participation status or test result. The NBCSP should lead to reductions in CRC mortality in Australia.