We describe the first pregnancy in Australia after fertility preservation by freezing of ovarian tissue for 7 years and subsequent autotransplantation in a woman treated for breast cancer, who was menopausal after chemotherapy. After transplantation and in-vitro fertilisation, she has a viable ongoing pregnancy.
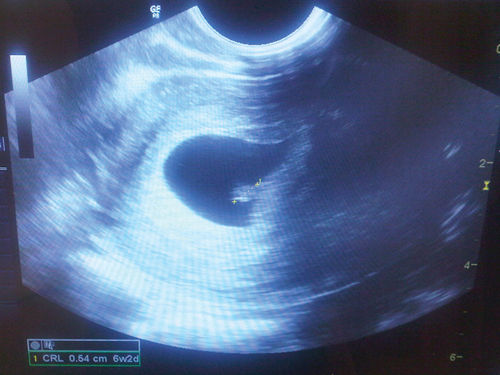
Fifteen days after oocyte retrieval, the woman had a β-hCG level of 259 IU/L and a progesterone level of 71.6 nmol/L. She continued vaginal progesterone treatment. At 5 weeks, she had a β-hCG level of 5140 IU/L and a progesterone level of 76.4 nmol/L. A vaginal ultrasound was performed at 6 weeks and 3 days, in November 2012, and a viable intrauterine pregnancy was confirmed, with a fetal heart rate of 120 beats/min (Box).
The pregnancy reported here is the first in Australia to occur after ovarian cryopreservation and autotransplantation. The first reported birth of a naturally conceived child after orthotopic transplantation of thawed frozen ovarian tissue was described in 2004.1 A pregnancy was subsequently reported where routine IVF with controlled ovarian hyperstimulation and transvaginal oocyte collection was carried out after orthotopic transplantation of ovarian tissue.2
After a review of fertility preservation options,3 we have now undertaken two attempts at ovarian autotransplantation using thawed cryopreserved ovarian cortex; the pregnancy described here represents the second of these. Our first attempt involved a woman who had pieces of ovarian cortex frozen at the age of 39 years in 2001 and subsequently underwent a natural menopause (serum FSH levels > 25 IU/L on two occasions). The ovarian tissue was reimplanted in 2009 and ovarian function returned, with FSH levels decreasing to premenopausal levels (7 IU/L and 12 IU/L) and oestrogen levels of up to 663 pmol/L, and with menstruation temporarily resuming. However, one attempt at controlled ovarian hyperstimulation was unsuccessful, and the woman declined further attempts.
A recent review reported that 17 babies had been conceived after ovarian cryopreservation and autotransplantation in 12 women in eight centres by the end of 2011.4 There has since been a report of a successful pregnancy in Germany.5 We therefore presume that the pregnancy reported here is one of the first 20 of its kind in the world, and only the second in a woman successfully treated for breast cancer.
It is not possible to express the success rate for autotransplantation of cryopreserved ovarian tissue, as we do not know how many attempts have been made to reimplant thawed frozen ovarian tissue in women (the denominator).6 The fact that both our attempts to date showed some degree of success, with the second achieving a pregnancy, may suggest this could become a preferred method of fertility preservation. It has the advantage that the ovarian tissue can be collected within 24 hours, no hormones need to be administered at the time of the cancer diagnosis, and the procedure takes less than half an hour and can be performed by any gynaecologist. The freezing technique is relatively straightforward, and storage facilities are available in every IVF unit. The process at the time of cancer diagnosis is also relatively inexpensive, as it only requires an operative laparoscopy and a few hours of a laboratory scientist’s time.
We performed the transplantation through a minilaparotomy, rather than laparoscopically, on the advice of our Israeli colleagues who had achieved two pregnancies by this technique.2 This enabled a much quicker procedure than laparoscopy and minimised tissue damage.
While technology needs to be developed and made available to increase the choices for fertility preservation for medical reasons,7 there is also a possible application of this technique in delaying or avoiding the menopause.
- 1. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364: 1405-1410.
- 2. Mierow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy [letter]. N Engl J Med 2005; 353: 318-321.
- 3. Kovacs GT, Rutherford AJ, Howlett D. Fertility preservation for women. O&G 2006; 8 (1): 12-14.
- 4. Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod 2012; 18: 59-67.
- 5. Isachenko V, Isachenko E, Keck G, et al. First live birth in Germany after re-transplantation of cryopreserved ovarian tissue: original device for initiation of ice formation. Clin Lab 2012; 58: 933-938.
- 6. Wallace WH, Kelsey TW, Anderson RA. Ovarian cryopreservation: experimental or established and a cure for the menopause? Reprod Biomed Online 2012; 25: 93-95.
- 7. Kovacs GT, Stern K. Reproductive aspects of cancer treatment: an update. Med J Aust 1999; 170: 495-497.





No relevant disclosures.