Diabetic retinopathy (DR) is one of the leading causes of preventable vision loss in Australia.1,2 The global prevalence of diabetes mellitus (DM) is on the rise, with 366 million (4.4% of the estimated world population) expected to be affected by the year 2030.3 In Australia, a nationwide cross-sectional survey in 2002 showed that the prevalence of diabetes in Australia had more than doubled since 1981. At the time of the survey, 8.0% of adult men and 6.8% of adult women living in Australia had diabetes, and 15.3% of people with diabetes had DR.4
DR is detected at similar rates in both Indigenous and non-Indigenous Australians with DM.5,6 However, the burden of DR is much higher among the Indigenous Australian population because of the higher prevalence of DM within this group. A recent eye health survey in Indigenous Australians showed the prevalence of self-reported diabetes to be more than eight times higher (37%) than in non-Indigenous Australians. This is a striking figure, as 30 years ago, only 0.03% of Indigenous people had diabetes.5
DR has little or no symptoms until vision loss develops, so regular DR screening is critical for early diagnosis and treatment.2,3 Vision loss can be prevented in up to 70% of people who are at risk through timely intervention.2,7 However, the rates of adherence to regular eye examinations in those with DR consistently fall below the recommended rate, and are as low as 50% in some studies.8,9 In particular, there is a significant shortfall in the delivery of DR screening to remote regions of Australia. This may have resulted from a shortage of service providers, obstacles in accessing centrally located specialist services and underutilisation of visiting services. Improving the availability of fundus cameras and training local staff in their use may help overcome some of the barriers to DR screening.
An ideal screening method requires acceptable sensitivity and specificity, and cost-effectiveness. A recent meta-analysis confirmed the use of retinal photography as a valid screening tool for DR in resource-poor settings.10 Our study was designed to evaluate the validity of single-field fundus photography as a screening tool for DR in remote Central Australian communities.
The design, recruitment process and baseline characteristics of the Central Australian Ocular Health Study (CAOHS) have previously been described in detail.11 The CAOHS took place in remote communities of Central Australia, excluding the relatively urbanised area of Alice Springs. The participants were recruited during once-weekly remote clinic visits over 36 months from 1 July 2005 to 30 June 2008. Ethics approval for the study was obtained from the Central Australian Human Research Ethics Committee according to the tenets of the Declaration of Helsinki. The aims of the study were explained to participants with the help of an interpreter when needed, and written informed consent was obtained.
All participants underwent detailed ocular examination. Baseline acuity was measured using a tumbling E acuity chart at 3 metres in a well lit room. An optometrist performed subjective refraction and determined refracted visual acuity. The optometrist performed a slit lamp examination of the anterior segment, followed by pupil examination using a hand torch. After an assessment of anterior chamber depth, the pupils were dilated using tropicamide 1.0% and phenylephrine 2.5% solution. The visiting ophthalmologist (T H) performed stereoscopic slit-lamp fundoscopy using a 90-dioptre fundoscopy lens. The presence and degree of DR was graded using the Early Treatment of Diabetic Retinopathy Study (ETDRS) adaptation of the modified Airlie House classification of DR12 by clinical comparison with standardised photographs (Box 1). The DR was graded as either no DR (level 10–13), minimal non-proliferative DR (NPDR) (level 14–19), mild NPDR (level 20–39), moderate NPDR (level 40–49), severe NPDR (level 50–59) and proliferative DR (PDR) (level 60–85). Clinically significant macular oedema (CSMO) was defined as any retinal thickening within 500 µm of the fovea associated with retinal thickening that is at least one disc area in size within one disc diameter of the fovea.
In line with many other papers reporting accuracy of screening photographs for DR, our paper has included data from both eyes to allow comparative assessment of accuracy of our study alongside others.10 The photos from individual patients were not assessed in pairs, but in a random order to minimise bias.
Various screening modalities for DR have been studied to meet the increased demand for screening, with variable rates of success. These include screening using mydriatic and non-mydriatic photography, and examination by different health professionals, including physicians, general practitioners and optometrists. To date, the ETDRS 7-field fundus photographs and ETDRS protocol are the only validated reference standard for detecting and staging of DR. Others have evaluated the validity of single-field fundus photography by comparing the accuracy of diagnosis against either a clinical gold standard of slit-lamp fundus examination13-16or an imaging gold standard of 7-field fundus photographs.17-19 Although dilated stereoscopic fundoscopy has an inherent weakness in that it cannot be validated or verified as there is no permanent record, it is by far the most commonly used method of DR evaluation in clinical practice. It allows clinicians to determine the presence of DR or VTDR. Thus, in our study, we set out to assess the validity of a screening method compared with standard clinical practice.
Any DR was detected in 163 eyes (23%) by clinical examination and in 162 eyes (23%) using fundus photography (Box 2, A). Of those with any DR detected by clinical examination, 52 (32%) had minimal NPDR, 63 (39%) had mild NPDR, 38 (23%) had moderate NPDR, one (1%) had severe NPDR, nine (6%) had PDR and 42 (26%) had CSMO.
VTDR was detected in 51 eyes (7%) on clinical examination and in 78 eyes (11%) using photo grading. Seven eyes with VTDR diagnosed clinically were not detected on photo screening, and 34 eyes were incorrectly diagnosed by photo grading as having VTDR (Box 2, B), leaving 44 eyes with VTDR (6%).
The sensitivity, specificity and kappa values for detecting any DR were 74% (95% CI, 67%–80%), 92% (95%CI, 90%–94%) and 0.67 (95% CI, 0.60–0.74; P < 0.0001), respectively (Box 3). The sensitivity and specificity for detecting VTDR were 86% (95% CI, 77%–96%), 95% (95% CI, 93%–97%) and 0.65 (95% CI, 0.55–0.76; P < 0.0001), respectively (Box 3).
The current National Health and Medical Research Council (NHMRC) Guidelines for the management of diabetic retinopathy recommend annual retinal examinations for Indigenous Australians, in contrast to every 2 years for the non-Indigenous population.20 Access to ophthalmology services is one major limitation to achieving screening goals in remote regions of Australia. A recent national population-based survey of eye health in the Indigenous Australian population redefined the gap in eye health between this group and other Australians. It is reported that 44% of Indigenous Australians had not had a diabetic eye screening in the past year.5
A systematic review of DR screening techniques supported retinal photography with mydriasis as the preferred method.21 Research comparing the accuracy of detecting DR using mydriatic and non-mydriatic fundus photographs showed an improvement in specificity with mydriasis.22 Other studies have shown that photos without mydriasis were often of poor quality and unable to be graded, especially in older patients and in the presence of media opacities.18,19 The higher failure rate of fundus photography in our study (11%; 86/792) compared with the reported failure rate of dilated fundus photography (4%–8%)14-16,18 may have resulted from a higher prevalence of media opacities, such as corneal scarring and cataract, among Indigenous Australians.23 Media opacity or small pupils would increase the likelihood of a false negative result, reducing the sensitivity of the screening photography. Thus we would argue that mydriasis is essential to improve diagnostic accuracy in this population group.
Most of the studies of fundus photographs to date are based largely on white populations.14,16,18,19 Previous studies evaluating fundus photography as a screening tool in Indigenous Australians were based on non-mydriatic fundus photographs with smaller sample sizes.24,25 To our knowledge, this is the first study that assesses the accuracy of detecting DR and VTDR in a large sample of Indigenous Australians using single-field dilated fundus photography compared with clinical examination. Our patient recruitment was somewhat limited by factors such as availability of instruments, and space and weight restrictions on light planes. However, this is unlikely to have introduced a systematic bias and we were able to obtain photographs of a representative sample of 706 eyes.
Cost analysis studies have demonstrated the potential cost-effectiveness of photo-screening in remote settings in maintaining both a high level of sight-years as well as being cheaper than visiting specialist programs.26,27
The NHMRC recommends that DR screening modalities need to be cost-effective and easy to administer, and to have a sensitivity of at least 60% and specificity levels of 90%–95%.20 The accuracy of the photo-screening in our study exceeds this minimum requirement, with sensitivities of 74% and 86% for detecting any DR and VTDR, respectively. Likewise, the specificities for detecting any DR and VTDR meet the NHMRC guideline of greater than 90%, at 92% and 95%, respectively. These values are comparable to the reported sensitivities and specificities of studies that compared diagnostic accuracy of single-field fundus photography with that of clinical examination. The sensitivities of detecting any DR have been reported from 38% to 96%, and specificities from 79% to 98%.13-16 Although definitions of VTDR vary in different studies, the sensitivities and specificities were higher for detecting VTDR, with ranges of 82%–100% and 70%–100%, respectively.13-16 In addition, the measure of reliability of inter-examiner and intra-examiner assessment (kappa coefficient κ) showed a moderate level of agreement (κ = 0.67 and 0.65 for any DR and VTDR, respectively).
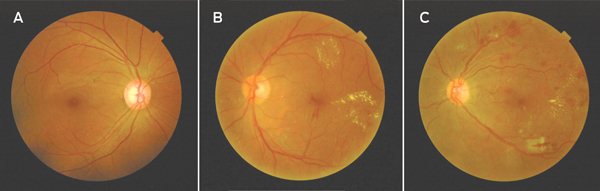
1 Fundus photographs (examples from study cohort) showing a normal fundus (A), clinically significant macular oedema (B) and proliferative diabetic retinopathy (C)
2 Accuracy of single-field 45-degree fundus photography in detecting any diabetic retinopathy (DR)* and vision-threatening diabetic retinopathy (VTDR) in 360 patients (706 eyes) using clinical slit-lamp fundoscopy as the gold standard
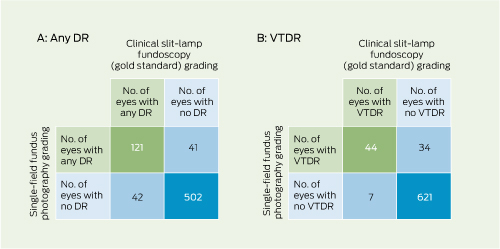
* Both of the categories “any DR” and “VTDR” include eyes with proliferative DR.
Received 8 April 2012, accepted 26 October 2012
- Janice J-Y Ku1
- John Landers2
- Tim Henderson3
- Jamie E Craig2
- 1 Sydney Hospital and Sydney Eye Hospital, Sydney, NSW.
- 2 Department of Ophthalmology, Flinders Medical Centre, Adelaide, SA.
- 3 Department of Ophthalmology, Alice Springs Hospital, Alice Springs, NT.
We would like to thank Renu Raju, Susan Wearne, Kathryn Billing, Tim Gray, Ilesh Patel, Jwu Jin Khong, John Chang, Shane Durkin and Douglas Parker, without whom this project would not be possible. We would also like to thank Vanessa Davies and Sarah Ford for their support.
This study is supported by the Ophthalmic Research Institute of Australia and the NHMRC Centres for Clinical Research Excellence.
- 1. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837-853.
- 2. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998; 105: 1801-1815.
- 3. Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82: 844-851.
- 4. Tapp RJ, Shaw JE, Harper CA, et al; AusDiab Study Group. The Prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care 2003; 26: 1731-1737.
- 5. Xie J, Arnold AL, Keeffe J, et al. Prevalence of self-reported diabetes and diabetic retinopathy in Indigenous Australians: the National Indigenous Eye Health Survey. Clin Experiment Ophthalmol 2011; 39: 487-493.
- 6. Landers J, Henderson T, Abhary S, Craig J. Prevalence and associations of diabetic retinopathy in Indigenous Australians within central Australia: the Central Australian Ocular Health Study. Clin Experiment Ophthalmol 2010; 38: 393-397.
- 7. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985; 103: 1796-1806.
- 8. Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology 2001; 108: 563-571.
- 9. Lee PP, Feldman ZW, Ostermann J, et al. Longitudinal rates of annual eye examinations of persons with diabetes and chronic eye diseases. Ophthalmology 2003; 110: 1952-1959.
- 10. Bragge P, Gruen RL, Chau M, et al. Screening for presence or absence of diabetic retinopathy: a meta-analysis. Arch Ophthalmol 2011; 129: 435-444.
- 11. Landers J, Henderson T, Craig J. Central Australian Ocular Health Study: design and baseline description of participants. Clin Experiment Ophthalmol 2010; 38: 375-380.
- 12. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98 (5 Suppl): 786-806.
- 13. Williams GA, Scott IU, Haller JA, et al. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology 2004; 111: 1055-1062.
- 14. Joannou J, Kalk WJ, Mahomed I, et al. Screening for diabetic retinopathy in South Africa with 60 degrees retinal colour photography. J Intern Med 1996; 239: 43-47.
- 15. Maberley D, Cruess AF, Barile G, Slakter J. Digital photographic screening for diabetic retinopathy in the James Bay Cree. Ophthalmic Epidemiol 2002; 9: 169-178.
- 16. Herbert HM, Jordan K, Flanagan DW. Is screening with digital imaging using one retinal view adequate? Eye (Lond) 2003; 17: 497-500.
- 17. Taylor DJ, Fisher J, Jacob J, Tooke JE. The use of digital cameras in a mobile retinal screening environment. Diabet Med 1999; 16: 680-686.
- 18. Pugh JA, Jacobson JM, Van Heuven WA, et al. Screening for diabetic retinopathy. The wide-angle retinal camera. Diabetes Care 1993; 16: 889-895.
- 19. Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol 2002; 134: 204-213.
- 20. National Health and Medical Research Council. Guidelines for the management of diabetic retinopathy. Canberra: Commonwealth of Australia, 2008. http://www.nhmrc.gov.au/guidelines/publications/di15 (accessed Jul 2012).
- 21. Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy--a systematic review. Diabet Med 2000; 17: 495-506.
- 22. Lawrence MG. The accuracy of digital-video retinal imaging to screen for diabetic retinopathy: an analysis of two digital-video retinal imaging systems using standard stereoscopic seven-field photography and dilated clinical examination as reference standards. Trans Am Ophthalmol Soc 2004; 102: 321-340.
- 23. Landers J, Henderson T, Craig J. Prevalence and associations of cataract in Indigenous Australians within central Australia: the Central Australian Ocular Health Study. Clin Experiment Ophthalmol 2010; 38: 387-392.
- 24. Diamond JP, McKinnon M, Barry C, et al. Non-mydriatic fundus photography: a viable alternative to fundoscopy for identification of diabetic retinopathy in an Aboriginal population in rural Western Australia? Aust N Z J Ophthalmol 1998; 26: 109-115.
- 25. Harper CA, Livingston PM, Wood C, et al. Screening for diabetic retinopathy using a non-mydriatic retinal camera in rural Victoria. Aust N Z J Ophthalmol 1998; 26: 117-121.
- 26. Lee SJ, McCarty CA, Taylor HR, Keeffe JE. Costs of mobile screening for diabetic retinopathy: a practical framework for rural populations. Aust J Rural Health 2001; 9: 186-192.
- 27. Maberley D, Walker H, Koushik A, Cruess A. Screening for diabetic retinopathy in James Bay, Ontario: a cost-effectiveness analysis. CMAJ 2003; 168: 160-164.





Abstract
Objective: To assess the accuracy of grading diabetic retinopathy (DR) using single-field digital fundus photography compared with clinical grading from a dilated slit-lamp fundus examination in Indigenous Australians living in Central Australia.
Design, setting and participants: Cross-sectional study comparing DR grades in participants with diabetes mellitus presenting for examination at remote community clinics from 1 July 2005 to 30 June 2008.
Main outcome measures: Sensitivity and specificity of grading using digital photography compared with the clinical gold standard of slit-lamp fundus examination.
Results: Of the 1884 participants recruited for the study, 1040 had self-reported diabetes mellitus and, of those, 360 had fundus photographs available (706 eyes) that were able to be graded. On clinical grading, 163 eyes had any DR and 51 eyes had vision-threatening DR (VTDR). The sensitivity and specificity for detecting any DR were 74% (95% CI, 67%–80%) and 92% (95% CI, 90%–94%), respectively. The sensitivity and specificity for detecting VTDR were 86% (95% CI, 77%–96%) and 95% (95% CI, 93%–97%), respectively.
Conclusion: Single-field digital fundus photography is a valid screening tool for DR in remote communities of central Australia and may be used to provide eye care services to this region with acceptable accuracy.