We report a case of inoperable metastatic parathyroid carcinoma involving life-threatening hypercalcaemia that failed to respond to standard therapy. Our review of available therapeutic modalities showed a paucity of evidence to guide management of patients with these rare tumours. This is the first report of the treatment of malignant hypercalcaemia secondary to parathyroid carcinoma using denosumab.
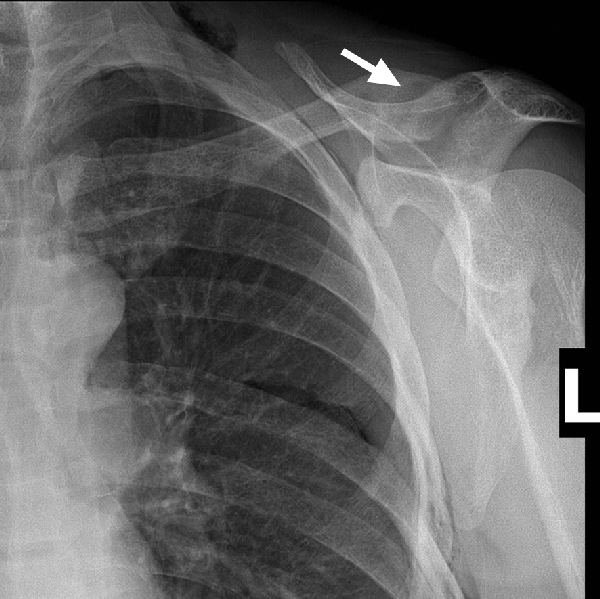
A 45-year-old man with no significant past medical history presented with a fractured clavicle and evidence of a radiolucent lesion (brown tumour) within the clavicle on x-ray, characteristic for hyperparathyroidism (Box 1). On further investigation, biochemical analysis showing raised serum calcium and parathyroid hormone (PTH) levels confirmed primary hyperparathyroidism. A thyroid ultrasound showed a 39 × 29 × 32 mm mass in the right lobe, suggestive of a tumour. A staging computed tomography (CT) scan of the neck and chest and a bone scan showed bone changes consistent with hyperparathyroidism throughout the axial and appendicular skeleton. There was no evidence of metastatic disease.
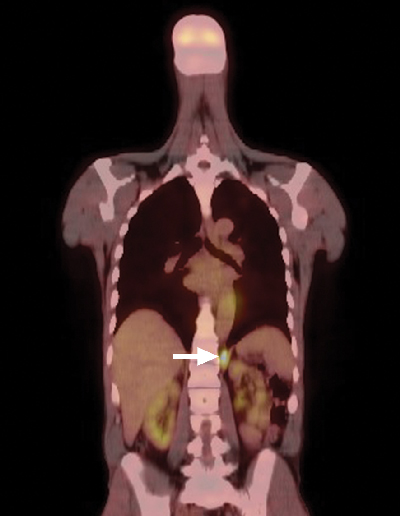
A positron emission tomography scan showed mildly increased fludeoxyglucose activity at the right thyroid bed, consistent with low-volume residual disease, and intense activity in the left retrocrural soft tissue (Box 2). Biopsy specimens were obtained from both sites and were found to be histologically consistent with parathyroid cancer. The patient underwent a planned resection of the known sites of disease, beginning with a right-sided neck dissection. Thereafter, he underwent a left thoracotomy, which showed more extensive pleural deposits and bulky metastatic disease over the diaphragm and crura, only amenable to debulking. A left pleural effusion, non-malignant on cytology, complicated his postoperative recovery.
We trialled denosumab as a means of achieving control of the resistant hypercalcaemia. We used loading doses, aiming to bring about rapid blockade of receptor activator of nuclear factor kappa B (RANK) ligand. The schedule was taken from a Phase II study of giant-cell tumours of bone, in which loading doses of denosumab 120 mg were given subcutaneously on Days 1, 8 and 15, with monthly maintenance therapy thereafter.1 In our patient, after the initial loading dose, the serum-corrected calcium level dropped to 2.66 mmol/L by Day 8. After a second dose, by Day 15 his calcium level was normal, at 2.33 mmol/L. Calculated urinary N-telopeptide levels were monitored to test for suppression of bone turnover. The baseline level was 431 nmol bone collagen equivalents (BCE)/mmol creatinine (RI, < 65 nmol BCE/mmol creatinine); by Day 15 it fell to < 20 nmol BCE/mmol creatinine, indicating rapid suppression of bone turnover. After two loading doses of denosumab, the patient’s serum calcium remained within the normal range for 4 months. His creatinine level also decreased to 102 μmol/L. Further treatment with denosumab was to be guided by the serum calcium level.
Despite control of the calcium level, the patient’s disease progressed and his PTH level rose to a maximum of 433 pmol/L. He became symptomatic, with a recurrent pleural effusion, anorexia and weight loss. Owing to a paucity of data on effective chemotherapeutic agents, the case was discussed at a departmental case review meeting and a regimen of carboplatin and gemcitabine was suggested, given its broad activity and use in carcinoma of unknown primary site.2 The patient completed six cycles of treatment with relative stability of the pleural effusion and retrocrural disease; however, his PTH level did not decrease. His physical condition deteriorated over 6 months after completion of chemotherapy, and he required one further dose of denosumab for calcium control. The patient died 1 year after his presentation with metastatic disease.
Parathyroid carcinoma is rare, accounting for less than 1% of cases of hyperparathyroidism. It is associated with a high rate of local and distant recurrence. The mainstay of treatment is surgical resection, even in metastatic disease. The disease is often refractory to medical management, leading to morbidity and mortality due to hypercalcaemia rather than metastatic disease; thus, an aggressive approach to resection has been advocated.3 Managing life-threatening hypercalcaemia and controlling a relatively indolent tumour pose a significant therapeutic challenge in inoperable disease.4
The efficacy of cinacalcet in the management of parathyroid cancer-related hypercalcaemia was assessed in an open-label single-arm study of 29 patients.5 Cinacalcet was commenced at 30 mg twice daily and titrated to 90 mg four times daily or until there was a response in the serum calcium. Over the 16-week study period, 62% of patients responded to therapy; the greatest response was seen in patients with the highest baseline calcium levels. PTH levels were also shown to decrease, but this was not clinically significant.
There is limited evidence for chemotherapy in the management of metastatic parathyroid carcinoma. Success using cyclophosphamide, 5-fluorouracil and dacarbazine has been reported, with normalisation of serum calcium and a complete response in pulmonary metastases after 12 cycles.6 There are several other case reports of agents that have demonstrated some efficacy, including dacarbazine as a single agent, the “MACC” regimen (methotrexate, adriamycin [doxorubicin], cyclophosphamide and CCNU [cyclonexyl-chloroethyl-nitrosourea, or lomustine]) and synthetic oestrogen therapy.4
Few strategies to manage resistant hypercalcaemia in parathyroid carcinoma have been published. One case report described immunisation using the bioactive section of PTH in a patient with resistant hypercalcaemia, with rapid improvement in symptoms and serum calcium. Autoantibodies to parathyroid hormone were identified in the patient’s serum within 4 weeks. The authors theorised that they prevented the binding of PTH to its receptors, thereby improving hypercalcaemia. There was no clinical or radiological evidence of tumour regression after 6 months of therapy.7
Denosumab is a fully human monoclonal IgG2 antibody that has a high affinity and specificity to bind RANK ligand, mimicking the effect of osteoprotegerin, which acts to competitively inhibit the binding of RANK ligand and RANK to inhibit osteoclast activation.8 Denosumab has been compared with zoledronic acid in the prevention of skeletal-related events, such as fractures, in patients with advanced malignancies in two randomised trials. In both, denosumab was associated with higher rates of hypocalcaemia and greater suppression of bone turnover markers.9,10 It is currently licensed in Australia for the treatment of osteoporosis and prevention of skeletal-related events in patients with metastatic breast and prostate cancer with bone metastases.
Preclinical data have shown that telomerase is active in parathyroid cancer cells and could be a potential therapeutic target. An in-vitro study of zidovudine, an antiretroviral agent, investigated its use in inhibiting telomerase. Zidovudine accumulated in malignant parathyroid cells in culture, which induced apoptosis. The effect was not observed in the adenomatous parathyroid cells, suggesting this could be considered as a targeted therapy.11 Studies in humans have not yet been done.
- 1. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010; 11: 275-280.
- 2. Pittman KB, Olver IN, Koczwara B, et al. Gemcitabine and carboplatin in carcinoma of unknown primary site: a phase 2 Adelaide Cancer Trials and Education Collaborative study. Br J Cancer 2006; 95: 1309-1313.
- 3. Mezhir JJ, Melis M, Headley RC, et al. Successful palliation of hypercalcemia secondary to metastatic parathyroid cancer: an unusual indication for hepatic resection. J Hepatobiliary Pancreat Surg 2007; 14: 410-413.
- 4. Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab 2001; 86: 485-493.
- 5. Silverberg SJ, Rubin MR, Faiman C, et al. Cinacalcet hydrocholoride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 2007; 92: 3803-3808.
- 6. Bukowski RM, Sheeler L, Cunningham J, Esselstyn C. Successful combination chemotherapy for metastatic parathyroid cancer. Arch Intern Med 1984; 144: 399-400.
- 7. Bradwell AR, Harvey TC. Control of hypercalcaemia of parathyroid carcinoma by immunisation. Lancet 1999; 353: 370-373.
- 8. Burkiewicz JS, Scarpace SL, Bruce SP. Denosumab in osteoporosis and oncology. Ann Pharmacother 2009; 43: 1445-1455.
- 9. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28: 5132-5139.
- 10. Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125-1132.
- 11. Falchetti A, Franchi A, Bordi C, et al. Azidothymidine induces apoptosis and inhibits cell growth and telomerase activity of human parathyroid cancer cells in culture. J Bone Miner Res 2005; 20: 410-418.





No relevant disclosures.