Weight loss and attenuated growth in height in children being treated with stimulant medication for attention deficit hyperactivity disorder (ADHD) is an area that has been plagued by controversy.1 The question of treatment affecting pubertal development is perhaps even more emotive as delay may be associated with significant psychopathology.2
The aims of this study were to investigate the influence of stimulant medication on growth rates and pubertal attainment of adolescent boys with ADHD and to compare these with a contemporaneous local cohort of community-sampled boys without ADHD.3-5 We have previously shown growth rates to be maximally attenuated during the first year, with a trend towards normalisation over 3 years of treatment.1 This suggests a progressive effect of stimulant medication on growth that plateaus at 3 years. Therefore, in order to standardise the effect of stimulant medication in this study, the subjects had a minimum of 3 years of continuous treatment.
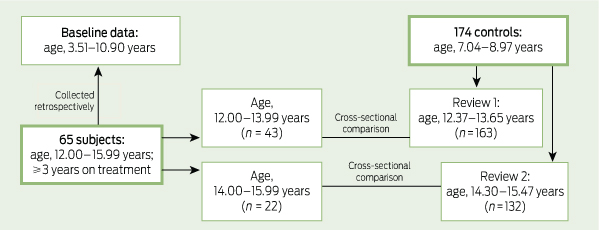
To monitor growth and development in boys receiving stimulant treatment for ADHD, we used cross-sectional measures of anthropometry and pubertal stage in adolescence and of anthropometry in childhood, and compared these data with similar data from childhood and adolescence in boys without ADHD (Box 1). Having anthropometry at two time points for each child meant that growth velocities could also be compared.
Height and weight measurements were taken at study enrolment, and a review of medical records was undertaken to obtain each boy’s baseline measurements (before starting treatment). Each boy was asked to identify his stage of puberty using Tanner staging with the help of pictures.6
For the controls, we used data from boys participating in the Nepean longitudinal study, an observational study originally designed to investigate the effects of birth size, body size and genes on blood pressure and bone mass.3-5 All were born at Nepean Hospital in western Sydney between 1989 and 1990 and had height and weight measurements available at 7–8 years of age and from one or both reviews at 13 and 15 years of age, when self-reported Tanner pubertal staging was also recorded.5
For subjects and controls, height was measured to the nearest 1 mm using a wall-mounted stadiometer, and weight was measured to the nearest 0.1 kg using electronic scales. All measurements were made without shoes and in light indoor clothing. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Measurements of height, weight and BMI were converted to age corrected z scores using the United States Centers for Disease Control and Prevention reference data using the LMS method.7,8
For analysis, the study cohort was stratified by age: 12.00–13.99 and 14.00–15.99 years. This was principally because the control data were clustered by age (Box 2). Growth parameters and pubertal stage of subjects and controls were compared using general linear modelling with age and baseline parameter z score (for growth data) as covariates.
When baseline measures were compared, the subjects before treatment were significantly younger than the controls aged 7–8 years (Box 2). There were no significant differences in height, weight or BMI after adjusting for age.
For the 43 subjects aged 12.00–13.99 years, the age-adjusted weights and BMIs were significantly lower than those of the controls (P = 0.003 for both) (Box 2). There were no significant differences in age-adjusted height velocity or weight velocity.
For the 22 subjects aged 14.00–15.99 years, after adjusting for age, the subjects were significantly shorter and lighter than the controls (P = 0.01 and P = 0.03, respectively) (Box 2). The mean age-adjusted weight velocity was significantly lower than the controls (P = 0.04) but there was no significant difference in age-adjusted height velocity.
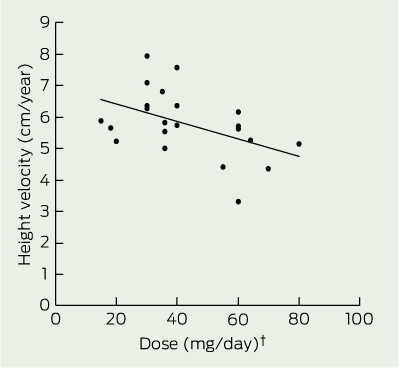
There was a significant inverse relationship between dose of medication (mg/day) and height velocity in the boys aged 14.00–15.99 years (r = 0.47; P = 0.03), which remained significant after adjusting for weight (P = 0.02) and age (P = 0.03) (Box 3). There was no significant dose effect on height velocity in the boys aged 12.00–13.99 years. The stage of puberty showed a significant correlation with the height velocity in the boys aged 12.00–13.99 years and those aged 14.00–15.99 years (r = 0.36; P = 0.02, and r = 0.45; P = 0.03, respectively), but there was no significant effect of the dose of medication on the stage of puberty in either age group. There was no independent effect of intermittent treatment or treatment duration on height velocity and no significant difference between dexamphetamine and methylphenidate.
An important weakness of the study was its use of pubertal self-staging, which could be associated with lack of precision or observer bias. Imprecision would tend to predispose to a type II error, but we found a significant difference between groups despite the methodological limitations. Further, if there were observer bias, the same direction of bias would be anticipated in the subjects and controls and would be unlikely to result in spurious differences between groups. Although pubertal staging by the doctor might give more reliable data, it is probable that few adolescent boys would have agreed to this. A study comparing physician ratings with self-reported pubertal staging in boys has found 91% concordance.9
We could find no longitudinal studies analysing the effect of stimulant medication on pubertal development. A cross-sectional study of 124 predominantly stimulant-treated boys with ADHD and 109 controls found no difference in the timing of the stages of puberty.10 Boys with ADHD in early puberty (Tanner stage 1–3) had shorter stature than boys without ADHD, but boys in late puberty (Tanner stage 4–5) had normal heights, implying catch-up growth during puberty. Although there was no evidence of pubertal delay, there is the suggestion of compromised height during the earlier stages of puberty in the boys with ADHD. A comparable study of girls by the same research team showed no delay in growth or pubertal development.11 However, no information was given on the duration of treatment.
Our previous study of prepubertal children showed that during the first 2.5 years of treatment with stimulant medication, the height velocity was about 1 cm/year slower than expected.1 We therefore anticipated, but did not find, slower growth rates in the younger boys. This could be due to the onset of the adolescent growth spurt, which has a peak height velocity of around 7–9 cm/year and occurs between Tanner stages 3 and 4.12 The physiological increase of up to 4 cm/year that is found in normal boys during puberty — and which occurs with a variable age of onset, reaching higher levels in early developers — would rapidly obscure in our cohort of boys with ADHD any residual early effect of the stimulant on growth. By contrast, the boys aged 14.00–15.99 years with ADHD showed slower growth, as indicated by their shorter stature compared with the controls. We postulate that this was due to a delay in the adolescent growth spurt. This delay would become most apparent after the majority of the boys without ADHD reached their peak height velocity. The significant inverse correlation between height velocity and dose of medication identifies this as a medication effect. It is likely that the pubertal delay observed in the ADHD subjects aged 14.00–15.99 years is similarly attributable to treatment with stimulant medication, given the well recognised correlation between peak height velocity and stage of puberty.12 Delay in the peak height velocity could have adverse social implications, for example, in the context of sport, where size and strength may be important.
Received 6 June 2012, accepted 5 November 2012
- Alison S Poulton1
- Elaine Melzer1
- Paul R Tait2
- Sarah P Garnett2
- Chris T Cowell2
- Louise A Baur2
- Simon Clarke2
- 1 Sydney Medical School Nepean, University of Sydney, Sydney, NSW.
- 2 The Children’s Hospital at Westmead, University of Sydney, Sydney, NSW.
We thank the children and their families for agreeing to participate in this study. The stimulant growth study was supported by the Australian Women and Childrens Research Foundation. The Nepean longitudinal study was supported by National Health and Medical Research Council (NHMRC) grant 206501 and a Meat and Livestock Australia grant. Sarah Garnett was supported by an NHMRC Australian Clinical Research Fellowship from 2007 to 2010 (457225) and is currently supported by a Cancer Institute NSW Fellowship from 2011 to 2013 (10/ECF/2-11). The funding organisations had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
No relevant disclosures.
- 1. Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: a characteristic pattern. J Paediatr Child Health 2003; 393: 180-185.
- 2. Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? J Am Acad Child Adolesc Psychiatry 2004; 436: 718-726.
- 3. Garnett S, Cowell C, Bradford D, et al. Effects of gender, body composition and birth size on IGF-I in 7-and 8-year-old children. Horm Res 2000; 525: 221-229.
- 4. Garnett SP, Baur LA, Srinivasan S, et al. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr 2007; 863: 549-555.
- 5. Garnett SP, Cowell CT, Baur LA, et al. Increasing central adiposity: the Nepean longitudinal study of young people aged 7–8 to 12–13 y. Int J Obes 2005; 2911: 1353-1360.
- 6. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45239: 13-23.
- 7. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990; 441: 45-60.
- 8. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002; 246.
- 9. Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics 1980; 666: 918-920.
- 10. Spencer TJ, Biederman J, Harding M, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry 1996; 3511: 1460-1469.
- 11. Biederman J, Faraone SV, Monuteaux MC, et al. Growth deficits and attention-deficit/hyperactivity disorder revisited: impact of gender, development, and treatment. Pediatrics 2003; 1115 Pt 1: 1010-1016.
- 12. Ferrandez A, Carrascosa A, Audi L, et al. Longitudinal pubertal growth according to age at pubertal growth spurt onset: data from a Spanish study including 458 children (223 boys and 235 girls). J Pediatr Endocrinol Metab 2009; 228: 715-726.





Abstract
Objective: To investigate the growth and pubertal attainment of boys with attention deficit hyperactivity disorder (ADHD) on stimulant medication.
Design, setting and participants: Longitudinal study of boys aged 12.00–15.99 years at recruitment in 2005–2011, with stimulant-treated ADHD for at least 3 years, attending three paediatric practices (subjects), compared with longitudinal data from 174 boys from the Nepean longitudinal study (controls).
Main outcome measures: Subjects’ growth parameters before treatment were compared with controls aged 7 or 8 years; growth parameters and longitudinal changes on treatment to ages 12.00–13.99 and 14.00–15.99 years were compared with controls reviewed at 13 and 15 years of age, respectively. The subjects’ pubertal staging and height velocity were related to their treatment history.
Results: Sixty-five subjects were recruited; mean duration of treatment was 6.3 ± 1.9 years. At baseline, their growth parameters were not significantly different from those of the controls after adjusting for age. Compared with the controls, after adjusting for current age and baseline growth parameter z score, subjects aged 12.00–13.99 years had significantly lower weight and body mass index (P < 0.01), and those aged 14.00–15.99 years had significantly lower height and weight (P < 0.05). At 12.00–13.99 years of age, the subjects were comparable to the controls in their pubertal development adjusted for age, but those aged 14.00–15.99 years reported significant delay (mean Tanner stage, 3.6 for subjects v 4.0 for controls; P < 0.05). The dose of medication was inversely correlated with the height velocity from baseline to 14.00–15.99 years of age (P < 0.05).
Conclusions: Prolonged treatment (more than 3 years) with stimulant medication was associated with a slower rate of physical development during puberty. To maintain adequate height velocity during puberty, we recommend keeping the dose as low as possible.