Amanita phalloides (“deathcap” or “whitecap”) is a cyclopeptide variety of mushroom that is responsible for more than 90% of mushroom-related fatalities; one mushroom cap can cause fulminant hepatic failure and death in an adult.1 Most reported A. phalloides poisonings occur in Europe. In Australia, most occur in Canberra as A. phalloides grows in the Australian Capital Territory, particularly in the older suburbs of Canberra (which has been attributed to imported oak trees). Cases have also been reported in Victoria. Public health campaigns are commonplace in the ACT in summer and autumn (“mushroom season”).
Amatoxin is the most potent hepatotoxin in cyclopeptide mushrooms; it irreversibly binds to RNA polymerase II, causing hepatic necrosis.1 The clinical features of A. phalloides poisoning are well described (Box 1). The most recent report of A. phalloides poisonings in Australia was from the ACT over 10 years ago, describing seven poisonings that occurred between 1988 and 1998.5 Since then, a chemically modified derivative of silibinin (silibinin-C-2',3-dihydrogen succinate, disodium salt [Legalon SIL, Madaus], referred to hereafter as silibinin) — a purported antidote to A. phalloides poisoning that is derived from the milk thistle Silybum marianum — has been stocked by hospitals in the ACT. In 2005, Canberra Hospital imported silibinin for use as an antidote through the Special Access Scheme of the Therapeutic Goods Administration because it is not registered for use in Australia. Sufficient stock was purchased to treat one patient for at least 96 hours at a dosage of 20 mg/kg/24 h, given in 6-hourly doses. This decision was made (despite a lack of good-quality evidence of efficacy) because there was no approved treatment in Australia, there was a plausible mechanism of action and some supporting evidence from animal studies, and silibinin is regarded as the standard of care in Europe. In 2010, the silibinin stock level at Canberra Hospital was increased so that there was sufficient stock to treat two patients and, in Sydney, Royal Prince Alfred Hospital also stocked it.
Onset of diarrhoea < 8 hours after mushroom ingestion or INR ≥ 6 on Day 4. These criteria are supported by a study of 27 patients (sensitivity 1.0 and specificity 0.68 for diarrhoea; sensitivity 1.0 and specificity 1.0 for INR)2 and a study of 10 patients (sensitivity 1.0 and specificity 1.0 for diarrhoea; sensitivity 0.71 and specificity 1.0 for INR),6 but they have been criticised because the supporting studies classified liver transplant recipients as deaths in their analysis.7
INR ≥ 2.5 and serum creatinine level > 106 μmol/L from Day 3. The reported predictive utility of these criteria is variable (sensitivity 1.0 and specificity 0.98 in a study of 198 patients;8 sensitivity 0.75 and specificity 0.90 in a study of 27 patients;2 sensitivity 0.57 and specificity 1.0 in a study of 10 patients6).
Over the 12-year study period, 12 patients presented with a history suggesting A. phalloides poisoning; they all developed clinical features of poisoning and were admitted to hospital (Box 2). The median age of the patients was 36 years (interquartile range, 27–51 years; range, 2–88 years) and eight patients were male. Eight of those with probable poisoning (based on clinical and mycological features) were not long-term residents of the ACT and seven were immigrants (six from Asia, one from Europe).
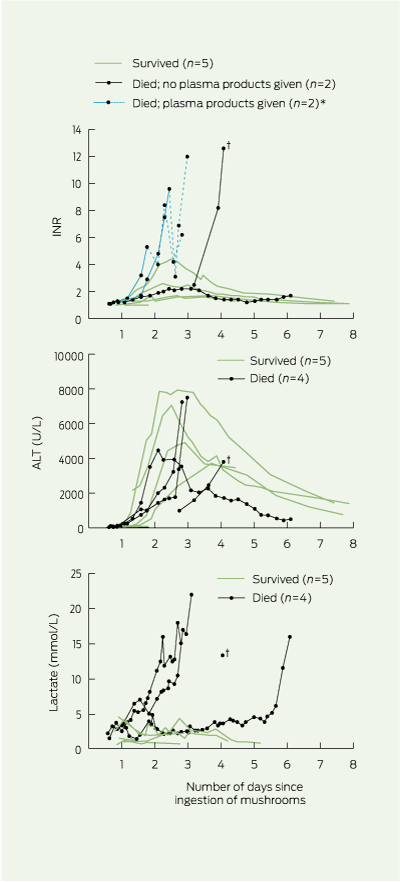
Temporal changes in INR, serum ALT levels and blood lactate levels are shown in Box 3. In most patients, marked changes in INR and ALT levels did not occur until at least 24 hours after mushroom ingestion. In two of the patients who died rapidly, the rate of increase of INR was more marked compared with that in survivors, but the same was not observed with ALT levels. The rate of increase in blood lactate levels was also more marked in patients who died compared with those who survived. The onset of increased INR and ALT levels appears to have been delayed for one of the patients who died and had not received silibinin (Patient G); however, the time of mushroom ingestion by this patient was imprecisely documented.
From our experience, elevated INR and blood lactate levels appeared to be the best prognostic markers. Other prognostic tools (listed in the Methods) did not provide useful advance warning of death — they met criteria only within several hours of death in the four patients who died. One death is notable because the INR values and ALT levels suggested resolution of hepatic injury, but the patient had progressive severe metabolic acidosis and hypoalbuminaemia and, 6 days after mushroom ingestion, developed an acute abdomen and subsequently died of cerebral oedema. The three patients who died rapidly with hepatic failure also had very severe lactic acidosis (Box 3). Severe gastrointestinal tract toxicity may be a significant contributory cause of death and may complicate liver transplantation.9 Toxicity progressed rapidly in two patients who were thought to have ingested the largest doses (about 8–10 mushrooms). Because of the small number of patients, it was not possible to explore the effectiveness of treatments.
This case series shows that A. phalloides poisoning is an ongoing public health concern. It is also the first report of silibinin use in Australia for A. phalloides poisoning. Despite availability of silibinin, multiple ingestions from 2000 to 2012 led to severe poisoning, including four deaths. Compared with data from the previous decade,5 the number of patients with hepatotoxicity more than doubled and the number of deaths quadrupled. However, the events were clustered and sporadic. People who do not reside in the ACT appear to be at higher risk, as do immigrants (six victims had recently arrived from Asia, where consumption of wild mushrooms is commonplace, and had limited English language skills). Those who consume mushrooms for recreational purposes may also be vulnerable.
The approach to treating patients with suspected A. phalloides poisoning includes prompt consideration of the diagnosis and identification of the mushroom if a fresh specimen or a sample of gastric contents is available. This requires liaison with a mycologist and performance of the Meixner test. The Meixner test is conducted by adding hydrochloric acid to a sample of mushroom placed on newspaper; a blue colour change suggests the presence of amatoxin, although false positive results have been reported.1 Treatment includes supportive care and gastrointestinal decontamination, and antidote use should be considered.10 Fluid resuscitation is required during the gastroenteritis phase to restore haemodynamic stability and maintain renal perfusion. Amatoxin is subject to enterohepatic recirculation, so multiple doses of activated charcoal should be given to limit reabsorption. Nasobiliary drainage has also been employed to reduce enterohepatic recirculation.11 Extracorporeal treatments have been trialled, but efficacy is inadequately quantified.
Electrolyte abnormalities should be corrected, and liver enzyme levels, INR, and lactate levels should be monitored for prognosticative purposes. Indications for liver transplantation are debated. Where possible, transplantation should be delayed until at least 2–4 days after poisoning to allow elimination of amatoxin to prevent graft poisoning.12 Early predictors of severe poisoning (but not necessarily death) may also help decision making regarding transfer to specialised liver units.
Multiple antidotes are currently used to treat patients with A. phalloides poisoning.1,10 The evidence of efficacy is limited for all treatments, largely due to the difficulties associated with human studies of an infrequent and sporadic type of poisoning. Two analyses of observational studies in human poisonings have supported the role of silibinin and acetylcysteine;10,13 however, positive findings are limited by potential publication bias.10,13 None of the treatments have been subjected to dose–response or controlled trials in humans. Many animal studies are of limited clinical relevance because treatments are not delayed. Further, in-vitro studies have demonstrated effects of benzylpenicillin, acetylcysteine and silibinin in human cultured hepatocytes,14-16 but not canine hepatocytes.17 A study in pigs demonstrated no beneficial effects of sili-binin, acetylcysteine, benzylpenicillin, cimetidine or thioctic acid administered 4 hours after poisoning.18 In our case series, four out of 10 patients with clinically significant poisoning died, despite the use of intravenous silibinin and other treatments in nine of them. The optimal doses and timing of antidotes, and the range of amatoxin ingestion for which antidotes are effective, are unknown.
1 Clinical features of acute Amanita phalloides poisoning1-4
2 Patients with a history suggesting Amanita phalloides poisoning, Australian Capital Teritory and New South Wales, 1999–2012*
Received 26 July 2012, accepted 3 December 2012
- Darren M Roberts1
- Michael J Hall2
- Morna M Falkland2
- Simone I Strasser3
- Nick A Buckley1,4
- 1 New South Wales Poisons Information Centre, Children’s Hospital at Westmead, Sydney, NSW.
- 2 Canberra Hospital, Canberra, ACT.
- 3 Australian National Liver Transplantation Unit, Royal Prince Alfred Hospital, Sydney, NSW.
- 4 Professorial Medicine Unit, Prince of Wales Hospital, University of New South Wales, Sydney, NSW.
We thank Emily Diprose for helpful comments on this article.
No relevant disclosures.
- 1. Berger KJ, Guss DA. Mycotoxins revisited: part I. J Emerg Med 2005; 28: 53-62.
- 2. Escudié L, Francoz C, Vinel JP, et al. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol 2007; 46: 466-473.
- 3. Giannini L, Vannacci A, Missanelli A, et al. Amatoxin poisoning: a 15-year retrospective analysis and follow-up evaluation of 105 patients. Clin Toxicol (Phila) 2007; 45: 539-542.
- 4. Krenová M, Pelclová D. Potential hepatotoxic and nephrotoxic substances reported to the Czech Toxicological Information Centre in the past 3 years. J Toxicol Clin Toxicol 2004; 42: 498.
- 5. Trim GM, Lepp H, Hall MJ, et al. Poisoning by Amanita phalloides (“deathcap”) mushrooms in the Australian Capital Territory. Med J Aust 1999; 171: 247-249. <MJA full text>
- 6. Ferreira R, Romãozinho JM, Amaro P, et al. Assessment of emergency liver transplantation criteria in acute liver failure due to Amanita phalloides. Eur J Gastroenterol Hepatol 2011; 23: 1226-1232.
- 7. Ganzert M, Felgenhauer N, Zilker T. Reassessment of predictors of fatal outcome in amatoxin poisoning: some critical comments. J Hepatol 2007; 47: 424-425.
- 8. Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol 2005; 42: 202-209.
- 9. Pinson CW, Daya MR, Benner KG, et al. Liver transplantation for severe Amanita phalloides mushroom poisoning. Am J Surg 1990; 159: 493-499.
- 10. Enjalbert F, Rapior S, Nouguier-Soulé J, et al. Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol 2002; 40: 715-757.
- 11. Madhok M, Scalzo AJ, Blume CM, et al. Amanita bisporigera ingestion: mistaken identity, dose-related toxicity, and improvement despite severe hepatotoxicity. Pediatr Emerg Care 2006; 22: 177-180.
- 12. Jaeger A, Jehl F, Flesch F, et al. Kinetics of amatoxins in human poisoning: therapeutic implications. J Toxicol Clin Toxicol 1993; 31: 63-80.
- 13. Poucheret P, Fons F, Doré JC, et al. Amatoxin poisoning treatment decision-making: pharmaco-therapeutic clinical strategy assessment using multidimensional multivariate statistic analysis. Toxicon 2010; 55: 1338-1345.
- 14. Magdalan J, Piotrowska A, Gomulkiewicz A, et al. Benzylpenicyllin and acetylcysteine protection from α-amanitin-induced apoptosis in human hepatocyte cultures. Exp Toxicol Pathol 2011; 63: 311-315.
- 15. Magdalan J, Ostrowska A, Piotrowska A, et al. Benzylpenicillin, acetylcysteine and silibinin as antidotes in human hepatocytes intoxicated with alpha-amanitin. Exp Toxicol Pathol 2010; 62: 367-373.
- 16. Magdalan J, Piotrowska A, Gomulkiewicz A, et al. Influence of commonly used clinical antidotes on antioxidant systems in human hepatocyte culture intoxicated with alpha-amanitin. Hum Exp Toxicol 2011; 30: 38-43.
- 17. Magdalan J, Ostrowska A, Piotrowska A, et al. Failure of benzylpenicillin, N-acetylcysteine and silibinin to reduce alpha-amanitin hepatotoxicity. In Vivo 2009; 23: 393-399.
- 18. Tong TC, Hernandez M, Richardson WH 3rd, et al. Comparative treatment of alpha-amanitin poisoning with N-acetylcysteine, benzylpenicillin, cimetidine, thioctic acid, and silybin in a murine model. Ann Emerg Med 2007; 50: 282-288.





Abstract
Objectives: To report the frequency and clinical outcomes of Amanita phalloides poisoning in the Australian Capital Territory and New South Wales, and the treatments used (including silibinin).
Design, setting and patients: Retrospective case series of patients admitted to public hospitals in Canberra and Sydney for suspected A. phalloides poisoning between 1999 and 2012 (identified from hospital records and calls to the New South Wales Poisons Information Centre).
Main outcome measures: Frequency of poisoning and the clinical outcomes.Results: Twelve patients presented with a history suggesting A. phalloides poisoning, 10 with probable poisoning and two with possible poisoning. Eight of those with probable poisoning developed significant hepatotoxicity and four died. Silibinin was administered to nine of those with probable poisoning (the other presented before 2005). Maintaining silibinin supply became a challenge during two clusters of poisoning. Eight of the patients with probable poisoning were not long-term residents of the ACT, and six were immigrants from Asia.
Conclusions: The mortality rate due to A. phalloides poisoning in this case series was high despite treatment according to current standards, including use of silibinin, and the frequency of hepatotoxicity was more than double that for the previous decade. Ongoing public health campaigns are required.