The antiprogesterone mifepristone combined with a prostaglandin analogue (misoprostol or gemeprost) is effective for terminating early pregnancy and has a favourable safety profile.1 Early medical abortion (EMA) regimens using 200 mg oral mifepristone and misoprostol have been endorsed by the Royal College of Obstetricians and Gynaecologists (RCOG) as an effective and appropriate method of terminating early pregnancy.2 Randomised controlled trials have shown that mifepristone 200 mg followed 24–48 hours later by misoprostol is as effective as surgical abortion in gestations up to 63 days, with complete abortion generally occurring in 93%–98% of cases, incomplete abortion in 1.1%–4.2%, and ongoing pregnancy in 0.2%–2.7%.3-10
Since its first registration in France in 1988, mifepristone has been registered in around 50 countries and is on the World Health Organization list of essential medicines.11 Mifepristone is currently an unapproved medicine in Australia, but can be accessed under the Authorised Prescriber provisions of the Therapeutic Goods Act 1989.12 Results for Authorised Prescriber use of mifepristone in Australia12-14 have been similar to those reported for other countries.1
Between 1 September 2009 and 31 August 2011, 13 345 EMAs were conducted at 15 MSIA clinics using the mifepristone–buccal misoprostol regimen (Box 1). Of the 12 968 women who had an EMA, 362 women (2.8%) had more than one EMA (Box 1). The mean age of the women was 28.4 years (SD, 6.8 years) (Box 1) and the mean length of gestation was 6.3 weeks (SD, 0.9 weeks) (Box 2).
For most EMAs (11 155/13 376, 83.4%; Box 3), follow-up information was obtained, primarily via a clinic visit, to confirm pregnancy termination. During the study, an extra 31 EMAs were recorded in the follow-up database (13 376) than in the patient demographics database (13 345; 0.2% variance). This discrepancy may be related to duplication of patient records in the follow-up database and carryover of follow-up records from a previously used treatment protocol for EMA.
The overall complication rate was low (519/13 345, 3.9%; Box 4). The EMA failure rate was 3.5% (465). Incomplete abortion requiring surgical aspiration occurred in 382 (2.9%) cases and continuing pregnancy in 83 (0.6%) cases. There were 16 cases of haemorrhage (0.1%). There were four cases (0.03%) of known and 21 cases (0.2%) of suspected infection, including one death from sepsis (< 0.01%). This woman suffered fever and flu-like symptoms about 6 days after taking mifepristone, but unfortunately did not seek medical advice, despite urging from family members. She died 9 days after taking mifepristone. Group A streptococcus (Streptococcus pyogenes) was identified from a vaginal swab and blood culture.
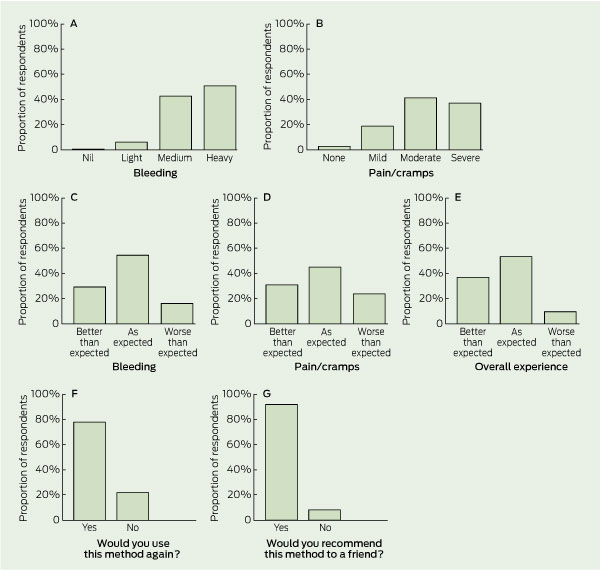
From April 2010 to August 2011, 10 093 EMAs were conducted. In 6755 of these EMAs, women attended follow-up and were asked to complete a seven-item questionnaire; if a woman had more than one EMA, she was asked to fill out a questionnaire for each EMA. Responses were available from 6381 women. Almost all of the women reported medium or heavy bleeding (5914/6330, 93.4%) and the majority reported moderate or severe pain/cramps (5001/6381, 78.4%) (Box 5, A and B). Most women said the bleeding (5226/6233, 83.8%), pain/cramps (4829/6340, 76.2%), and overall experience (5660/6265, 90.3%) were as expected or better than expected (Box 5, C–E). Most women reported that they would choose the EMA method again (4939/6335, 78.0%) and would recommend it to a friend (4770/5196, 91.8%) (Box 5, F and G).
The low failure rate (3.5%) with mifepristone in our study was similar to that reported for mifepristone in randomised controlled trials (failure rates, 2%–7%)3-10 and in other Australian Authorised Prescriber Schemes (failure rates, 2.9%–5.6%).13,14 Notably, the rate of surgical intervention for incomplete abortion declined from 4.3% during the first 3-month period of our study to 1.6%–2.6% during subsequent 3-month periods. We propose that as clinic staff gained experience with the EMA method, they felt more comfortable with expectant management rather than proceeding directly to surgical intervention.
In our study, there were four cases of known infection reported (0.03%), including one death from sepsis. Infection following medical termination of pregnancy is reported to occur in 0.02%–0.92% of cases.15,16 Death resulting from infection and fatal toxic shock after medical abortion with mifepristone is a known, but very rare, risk (1.1/100 000).15 Although the RCOG2 recommends universal prophylactic antibiotics effective against C. trachomatis and anaerobes to reduce the risk of infection after medical abortion, the WHO17 does not. At MSIA, all women are screened for C. trachomatis, unless they decline, and for other STIs based on a risk assessment. MSIA has upgraded its warnings to women regarding serious infections and also now provides an opt-in SMS service that reminds women 3–5 days after their visit about the after-care phone number and signs and symptoms of complications.
The regimen we used is similar to those recommended by the WHO17 and the RCOG,2 with buccal administration of misoprostol. Vaginal misoprostol has been associated with deaths from toxic shock syndrome in the United States18 and some women may prefer buccal, rather than vaginal, administration. In addition, oral misoprostol is not sufficiently effective beyond 49 days of gestation.1,2,17 Randomised controlled trials have shown mifepristone with buccal misoprostol to have efficacy similar to or greater than mifepristone with vaginal7 or oral19 misoprostol for terminating pregnancies up to 56 and 63 days, respectively. Our study provides further support for using 200 mg of mifepristone, which is lower than the 600 mg dose approved in the US and Europe.1 Randomised controlled trials directly comparing the two mifepristone doses have shown similar efficacy for the 200 mg and 600 mg doses.3,6 A Cochrane meta-analysis also found no difference in failure rates between the 200 mg and 600 mg doses.20
In our study, all women were informed of the need for mandatory follow-up to confirm pregnancy termination; contact with most women was achieved. Those who did not attend the clinic were telephoned and, if telephone contact was not possible, a registered letter was sent (the letter included a pregnancy test kit to facilitate home-testing). Despite these efforts, follow-up information was missing for around 17% of women. The loss to follow-up in our real-world setting of mifepristone use was higher than that observed in rigorous clinical trial settings (1.7%–8.6%).3-5,8,9,21 As might be expected in clinical practice, not all patients may return for follow-up, even if instructed to attend a follow-up visit to assess treatment outcome. Given the emotional issues surrounding abortion, women who felt the abortion was complete and had no physical complications may have been reluctant to return to the clinic. While this is of concern, a recent study using early telephone follow-up after medical abortion to determine the need for further evaluation found that women who initially had not required evaluation returned to the clinic of their own accord if they experienced problems, such as bleeding or continuing pregnancy.21 This finding suggests that women who experience adverse effects or feel that the abortion is not complete are likely to seek appropriate medical care. Indeed, in our study, about one-third of women did call the MSIA after-care service about issues such as pain or bleeding. While many women required reassurance only, some callers were referred to the clinic or a hospital for further assessment. In addition, it is possible that some women received follow-up care elsewhere. Not all women with complications, however, will decide to seek follow-up care and, in very rare cases (eg, one out of 13 345 cases in our study), this can have serious consequences. Understandably, such cases can raise concerns about the safety of home-based versus clinic-based administration of misoprostol. Although misoprostol could be administered in a clinic under medical supervision, home-based administration of misoprostol has been associated with a low complication rate, is preferred by women, and is common practice in France and the US.1,22 Further, clinic-based administration of misoprostol would not necessarily enhance outcomes as medical abortion complications can occur hours or days after misoprostol is administered.
Overall, there was a high level of satisfaction with the mifepristone–buccal misoprostol regimen used, with most women who completed the study questionnaire reporting the bleeding, pain/cramps, and overall experience to be as they had expected or better than expected. Satisfaction with the EMA method used in our study was similar to that reported in other studies.1,23,24 Notably, there has been a significant uptake of medical abortion in our clinics, with about a third of women requesting an abortion up to 63 days of gestation opting for the medical method (data not shown).
Our mifepristone–buccal misoprostol EMA method could be used outside a hospital setting. The diagnosis and initiation of treatment (mifepristone) can be conducted in a clinic and the second part of the treatment (misoprostol) can be carried out by the woman at home, with 24-hour access to after-care services to manage any issues. This arrangement, along with the 24–48-hour window for misoprostol administration, allows the abortion process to take place in the privacy of the woman’s home and at the time of her choosing, factors known to be of importance to women undergoing an abortion.1,23,24 The clinic- and home-based nature of the mifepristone–buccal misoprostol regimen makes it feasible for use in regional centres of Australia. These centres may have smaller hospitals than urban areas, but have the facilities and staff to manage spontaneous miscarriage.24 The MSIA clinics are located in major metropolitan areas and women are provided with discharge letters. Any woman contacting the after-care service can be referred back to the MSIA clinic or to her local doctor or, if necessary, a nearby accident and emergency facility. In clinical practice, proximity to emergency services should be evaluated when considering the use of the mifepristone–buccal misoprostol regimen. The potential need for access to appropriate 24-hour emergency help could limit the use of this regimen in more remote areas of Australia.
1 Demographic data for women undergoing early medical abortion (EMA) with mifepristone–buccal misoprostol
2 Length of gestation for women undergoing early medical abortion (EMA) with mifepristone–buccal misoprostol (n = 13 345)
* Figures represent number of EMAs, except those relating to length of gestation. |
|||||||||||||||
3 Follow-up contact for women undergoing early medical abortion (EMA) with mifepristone–buccal misoprostol
4 Reported complications for women undergoing early medical abortion (EMA) with mifepristone–buccal misoprostol
Received 14 February 2012, accepted 6 August 2012
- Philip Goldstone1
- Jill Michelson2
- Eve Williamson1
- Marie Stopes International Australia, Melbourne, VIC.
This study was sponsored by Marie Stopes International Australia, a not-for-profit, non-governmental organisation. The sponsor did not impose any impediment, directly or indirectly, on the publication of our results. We acknowledge the independent medical writing assistance of Justine Southby and Julie Ely of ProScribe Medical Communications, funded from an unrestricted financial grant from MSIA. We also acknowledge the contributions to the study from the Marie Stopes Study Group: Ivana Borsky, Harry Cohen, Nicole Gastaldin, Janelle Hall, Katerina Lagios, Gary Lubransky, Alex MacPherson, Kevin Pedemont, Andrew Perry, Rebecca Quake, Charles Russell-Smith, Robin Tideman, and Ying Zhou.
Philip Goldstone is an employee of, and Jill Michelson and Eve Williamson are consultants to, Marie Stopes International Australia.
- 1. Fiala C, Gemzel-Danielsson K. Review of medical abortion using mifepristone in combination with a prostaglandin analogue. Contraception 2006; 74: 66-86.
- 2. Royal College of Obstetricians and Gynaecologists. The care of women requesting induced abortion. Evidence-based clinical guideline number 7. London: RCOG, 2011. http://www.rcog.org.uk/files/rcog-corp/Abortion%20guideline_web_1.pdf (accessed Jun 2012).
- 3. World Health Organization Task Force on Post-ovulatory Methods of Fertility Regulation. Comparison of two doses of mifepristone in combination with misoprostol for early medical abortion: a randomised trial. BJOG 2000; 107: 524-530.
- 4. Bartley J, Brown A, Elton R, Baird DT. Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation. Hum Reprod 2001; 16: 2098-2102.
- 5. Creinin MD, Fox MC, Teal S, et al. A randomized comparison of misoprostol 6 to 8 hours versus 24 hours after mifepristone for abortion. Obstet Gynecol 2004; 103: 851-859.
- 6. McKinley C, Thong KJ, Baird DT. The effect of dose of mifepristone and gestation on the efficacy of medical abortion with mifepristone and misoprostol. Hum Reprod 1993; 8: 1502-1505.
- 7. Middleton T, Schaff E, Fielding SL, et al. Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period. Contraception 2005; 72: 328-332.
- 8. Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol 2 days after mifepristone 200 mg for abortion up to 63 days of pregnancy. Contraception 2002; 66: 247-250.
- 9. Schaff EA, Fielding SL, Westhoff C, et al. Vaginal misoprostol administered 1, 2, or 3 days after mifepristone for early medical abortion: a randomized trial. JAMA 2000; 284: 1948-1953.
- 10. Tang OS, Chan CC, Ng EH, et al. A prospective, randomized, placebo-controlled trial on the use of mifepristone with sublingual or vaginal misoprostol for medical abortions of less than 9 weeks gestation. Hum Reprod 2003; 18: 2315-2318.
- 11. Gibson L. WHO puts abortifacients on its essential drug list. BMJ 2005; 331: 68.
- 12. de Costa CM, Russell DB, de Costa NR, et al. Early medical abortion in Cairns, Queensland: July 2006 – April 2007. Med J Aust 2007; 187: 171-173. <MJA full text>
- 13. de Costa CM. Use of mifepristone for medical abortion in Australia, 2006–2009. Med J Aust 2011; 194: 206-207. <MJA full text>
- 14. Mulligan E, Messenger H. Mifepristone in South Australia — the first 1343 tablets. Aust Fam Physician 2011; 40: 342-345.
- 15. Henderson JT, Hwang AC, Harper CC, Stewart FH. Safety of mifepristone abortions in clinical use. Contraception 2005; 72: 175-178.
- 16. Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: A review of the literature. Contraception 2004; 70: 183-190.
- 17. World Health Organization. Safe abortion: technical and policy guidance for health systems. 2nd ed. Geneva: WHO, 2012.
- 18. Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome sssociated with Clostridium sordellii after medical abortion. New Engl J Med 2005; 353: 2352-2360.
- 19. Winikoff B, Dzuba IG, Creinin MD, et al. Two distinct oral routes of misoprostol in mifepristone medical abortion: a randomized controlled trial. Obstet Gynecol 2008; 112: 1303-1310.
- 20. Kulier R, Kapp N, Gulmezoglu AM, et al. Medical methods for first trimester abortion. Cochrane Database Syst Rev 2011; (11): CD002855. DOI: 10.1002/14651858.CD002855.pub4.
- 21. Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception 2010; 81: 143-149.
- 22. Ngo TD, Park MH, Shakur H, Free C. Comparative effectiveness, safety and acceptability of medical abortion at home and in a clinic: a systematic review. Bull World Health Organ 2011; 89: 360-370.
- 23. Fiala C, Winikoff B, Helstrom L, et al. Acceptability of home-use of misoprostol in medical abortion. Contraception 2004; 70: 387-392.
- 24. de Costa CM. Medical abortion for Australian women: it’s time. Med J Aust 2005; 183: 378-380. <MJA full text>





Abstract
Objective: To describe the use of mifepristone in combination with buccal misoprostol in women undergoing an early medical abortion (EMA) in Australia.
Design, setting and participants: Retrospective, observational study of 13 345 EMAs (gestational age ≤ 63 days) conducted at 15 Marie Stopes International Australia clinics between 1 September 2009 and 31 August 2011.
Intervention: Oral mifepristone 200 mg, administered at the clinic, followed 24–48 hours later by buccal misoprostol 800 µg, self-administered at home.
Main outcome measure: Failure rate (proportion of women with an incomplete abortion requiring surgical aspiration or a continuing pregnancy).
Results: Pregnancy termination follow-up information was available for 83.4% (11 155/13 376) of EMAs. From the patient demographic database, the EMA failure rate was 3.5% (465/13 345). Of these, most (382; 2.9% of total) were incomplete abortions requiring surgical aspiration, and 83 (0.6% of total) were continuing pregnancies. Haemorrhage (16; 0.1%) and known or suspected infection (25; 0.2%) were infrequent. One woman, who did not seek follow-up despite signs of infection, died from sepsis (< 0.01%). In 6755 EMAs with clinic follow-up from April 2010 to August 2011, 6381 women participated in a survey. Most reported medium or heavy bleeding and moderate or severe pain/cramps; most also reported that bleeding, pain/cramps and their overall experience were as expected or better than expected.
Conclusions: Mifepristone, with buccal misoprostol self-administered at home, for EMA up to 63 days of gestation had a low failure rate, was well accepted, and provided an effective treatment option with a favourable safety profile for women seeking an abortion in Australia.