In the early years of transplantation, organs were retrieved from patients who were considered to have died by traditional cardiopulmonary criteria (now known as donation after cardiac death [DCD]) or from live donors.1 The introduction of diagnostic criteria for brain death provided a more practical and controlled pathway to organ donation.2 Donation after brain death (DBrD) allowed organ retrieval without prior cessation of circulation, thus minimising warm ischaemic time and enhancing organ function in the recipient.
Demand for organs for transplantation has grown and outstrips supply.3 In recent years some countries have successfully re-implemented DCD to increase overall donor numbers.4 Long-term survival of grafts and recipients after DCD kidney and lung transplantation are equivalent to those after DBrD,5-7 but DCD liver grafts are still subject to higher risk.8 Currently, however, DCD is under-used in Australia and practised at few institutions. Given a growing body of evidence,9 there appears to be considerable potential for DCD to expand the donor pool.
In Australia, a resurgence of interest in DCD has come with concerns over public and staff perceptions, the possibility of a conflict of interest for clinicians when considering intensive care unit (ICU) treatment withdrawal,10-12 the impact on organ function and a potential reduction in the number of DBrD donors resulting from clinicians or families choosing DCD over DBrD.13
In 2004 and 2005, animal models of lung transplantation after cardiac death were investigated at the Alfred Hospital with promising results.14 Coincidentally, in 2005, organ donation was requested by the family of a patient who had severe brain injury but was not brain dead. The family’s request could not be facilitated and the patient died without becoming a donor. A complaint was subsequently lodged about the hospital’s inability to facilitate organ donation. This provided the impetus to gather interested individuals together, write a guideline and develop an implementation plan for DCD. Although national guidelines for DCD have subsequently been published,15 at the time, there were none.
Medical suitability: In addition to pre-existing selection criteria for organ donors, only ventilated patients within the ICU were considered. Initially, an age limit of 55 years was applied. However over the next 5 years, this was increased from 55 to 65 years and then to 75 years.16 Initially, only lung donation was considered because of a lack of on-call access to renal retrieval services. In 2007, kidney donation, and in 2008, donation of any other suitable organ (eg, liver or pancreas) were included.
Logistics: Considerable planning went into logistics. Processes and roles of key staff are summarised in Box 1. A team meeting with all relevant ICU, theatre, surgical, anaesthetic, medical, nursing and support staff was planned before withdrawing cardiopulmonary support to discuss all aspects of the process. Follow-up meetings were held 24–48 hours after every case of DCD during the first 2 years, and then when required thereafter.
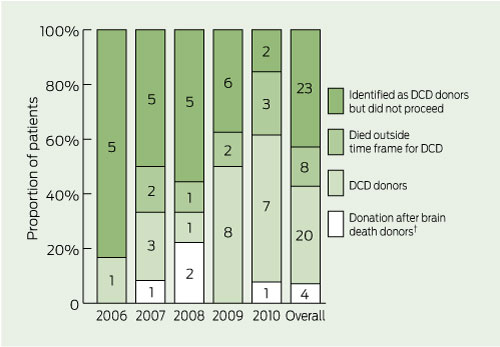
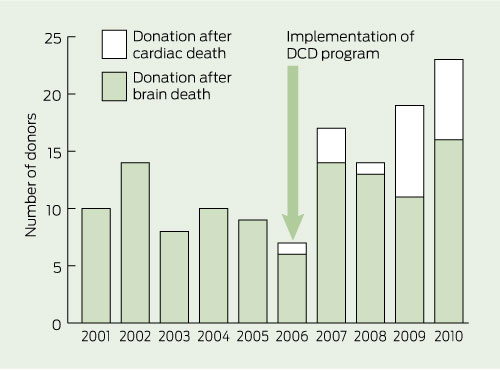
Box 2 shows the total number of donation after brain death and DCD donors over the past 10 years at the Alfred Hospital, with DCD accounting for eight of the 19 donors in 2009 and seven of the 23 donors in 2010. During the first 5 years of the program, the Alfred Hospital contributed 20 of the 54 DCD donors in Victoria, comprising 12.4% (20/161) of all DCD donors in Australia. There was no concurrent reduction in the number of DBrD donors. Four patients became DBrD donors while being considered for DCD. In one case, a patient became brain dead, but the DCD pathway was followed owing to family insistence. One DCD donor was probably brain dead, but this was not recognised before withdrawal of cardiorespiratory support. Box 3 shows actual DCD donors per year as percentages of all patients for whom DCD was considered.
Of Australia’s total organ donors, 6.6% (80/1215) were identified at the Alfred Hospital in 2006–2010, compared with 5.1% (51/992) in 2000–2005, before the DCD program was implemented (P = 0.23). Overall admissions to the ICU at the Alfred Hospital did not change significantly over the 10 years, remaining stable at about 2000 per year. Implementation of the DCD program resulted in 62 organ transplants, 17 of these organs came from the four donors who were brain dead and who were identified through the DCD program (Box 4).
Acceptance by medical and nursing staff has been progressive and there is evidence of a “learning curve” with the DCD program. Each year a larger proportion of patients in whom DCD was considered had cardiorespiratory support withdrawn in anticipation of DCD (Box 3 and Box 5). However, the ability to predict who will die within a time frame permitting successful donation remained unchanged.
The Alfred Hospital DCD guideline continues to differ slightly from the national guideline for DCD produced in 201015 (eg, in not allowing antemortem interventions such as heparin infusion or bronchoscopy). Victoria remains reliant on individual hospitals developing policies which best suit local practice rather than applying a statewide policy.
Although absolute numbers of donors are relatively small, the potential impact on available organs for transplantation is high. Our study shows that introduction of DCD at one tertiary hospital can lead to increased numbers of organs available for transplantation without any reduction in DBrD donations. The National protocol for donation after cardiac death, published in 2010,15 now provides a framework to develop similar programs. We believe all hospitals in Australia should investigate their potential for DCD.
3 Outcomes of organ donation from potential donation after cardiac death (DCD) donors at the Alfred Hospital from 2006 when the DCD program was implemented until 2010*
4 Organs and tissue successfully retrieved and transplanted as a result of the Alfred Hospital donation after cardiac death (DCD) program
Transplants from DBrD donors identified through the DCD program |
|||||||||||||||
5 Reasons patients considered for donation after cardiac death (DCD) did not go on to become donors
Received 6 August 2011, accepted 22 January 2012
- Tim G Coulson1
- David V Pilcher1,2
- Shena M Graham1
- Gregory I Snell1
- Bronwyn J Levvey1
- Steve Philpot1
- Alvin Teo1
- Andrew R Davies1
- 1 Alfred Hospital, Melbourne, VIC.
- 2 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
We thank the Alfred Hospital medical, nursing and administrative staff, the legal support team, the DonateLife team, staff of the Victorian Institute of Forensic Medicine and the donor families.
David Pilcher is a Medical Advisor to DonateLife in Victoria.
- 1. Linden PK. History of solid organ transplantation and organ donation. Crit Care Clin 2009; 25: 165-184, ix.
- 2. Potts JT, Herdman R. Non-heart-beating organ transplantation: medical and ethical issues in procurement. Washington, DC: National Academies Press, 1997.
- 3. Excell L, Hee K, Russ G. Australia and New Zealand Organ Donation Registry 2010 Report. Adelaide: ANZOD Registry, 2010. http://www.anzdata.org.au/anzod/v1/AR-2010.html (accessed Sep 2011).
- 4. Mañalich R, Paez G, Valero R, Manyalich M. IRODaT: the International Online Registry for Organ Donation and Transplantation 2007. Transplant Proc 2009; 41: 2030-2034.
- 5. Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010; 376: 1303-1311.
- 6. Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant 2008; 8: 1282-1289.
- 7. De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010; 139: 1306-1315.
- 8. Reich DJ, Hong JC. Current status of donation after cardiac death liver transplantation. Curr Opin Organ Transplant 2010; 15: 316-321.
- 9. Snell GI, Levvey B, Oto T, et al. Effect of multiorgan donation after cardiac death retrieval on lung performance. ANZ J Surg 2008; 78: 262-265.
- 10. Sayegh MH, Carpenter CB. Transplantation 50 years later--progress, challenges, and promises. N Engl J Med 2004; 351: 2761-2766.
- 11. Snell GI, Levvey BJ, Williams TJ. Non-heart beating organ donation. Intern Med J 2004; 34: 501-503.
- 12. Mandell M, Zamudio S, Seem D, et al. National evaluation of healthcare provider attitudes toward organ donation after cardiac death. Crit Care Med 2006; 34: 2952-2958.
- 13. Nath J, Mellor SJ. Organ transplantation after cardiac death. Lancet 2011; 377: 203.
- 14. Snell GI, Oto T, Levvey B, et al. Evaluation of techniques for lung transplantation following donation after cardiac death. Ann Thorac Surg 2006; 81: 2014-2019.
- 15. National Health and Medical Research Council. National protocol for donation after cardiac death. Canberra: Australian Organ and Tissue Donation and Transplantation Authority, 2010. http://www.donatelife.gov.au/the-authority/publications (accessed Sep 2011).
- 16. Snell GI, Esmore DS, Westall GP, et al. The Alfred Hospital lung transplant experience. Clin Transpl 2007: 131-144.





Abstract
Objectives: To describe the design, development and implementation of an organ and tissue donation after cardiac death (DCD) program, evaluate its success and assess its impact on tissue and organ availability and the number of donors after brain death.
Design, participants and setting: Prospective collection of patient characteristics and outcomes for actual and potential donors from 2000 to 2010, thus including the 5 years after the implementation of a DCD program at a major Australian tertiary hospital in 2006.
Main outcome measures: The number and type of donors before and after implementation of the DCD program, and subsequent numbers of solid organ and tissue donations.
Results: The DCD program was associated with an increase in overall donor numbers. There were 80 donors (20 DCD and 60 donation after brain death [DBrD]) after 2006, compared with 51 DBrD donors in the previous 5 years. Four of the DBrD donors were patients who were initially considered for DCD. DCD accounted for eight of the total 19 donors in 2009 and seven of the total 23 donors in 2010. There were 62 solid organ and 35 tissue and cornea transplants as a result of the DCD program.
Conclusions: Successful implementation of a DCD program is possible and has led to an increase in overall donor numbers and organs transplanted without any reduction in DBrD donors. The widespread implementation of DCD across Australia may help reduce the shortfall of organs for transplantation.