The administration of pandemic (H1N1) 2009 influenza A vaccine as part of the pandemic response presented challenges for vaccine safety authorities internationally. The monovalent H1N1 vaccine used in Australia was an egg-culture-based, inactivated, split-virion vaccine, with a demonstrated safety profile similar to that of the seasonal trivalent influenza vaccine (TIV).1 Postmarketing active surveillance through Phase IV clinical trials is required for potential rare adverse events following immunisation (AEFI), such as Guillain-Barré syndrome (GBS).2
In 1976–1977, H1N1 immunisation was associated with GBS in the United States.3 A relative incidence (RI) of 7.6 (95% CI, 6.7–8.6) was reported. This correlated with an estimated excess of nine cases of GBS per million vaccinations in the 42 days after immunisation. Two subsequent studies using the United Kingdom General Practice Research Database (1990–2005) found no association between influenza vaccine and GBS.4,5 A Canadian study between 1992 and 2004 demonstrated a small but statistically significant temporal association between influenza vaccination and subsequent hospital admission for GBS (RI, 1.45; 95% CI, 1.05–1.99), but reported no increase in GBS at the population level after a mass influenza vaccination in Ontario beginning in 2000.6 The estimated frequency of influenza illness-related GBS is four to seven times higher than the estimated frequency for influenza vaccine-associated GBS.7
A literature review (1950–2008) found that, with rare exceptions, associations between vaccines and GBS have been only temporal with little evidence to support a causal association.8 Challenges for studies examining the epidemiology of GBS include difficulties in case ascertainment and diagnostic uncertainty in identified cases. Additionally, in the absence of influenza vaccine registries, there is often uncertainty in determining the number of vaccines administered. Our study aimed to explore any potential association between monovalent H1N1 vaccine and/or H1N1-containing TIV, and the occurrence of GBS.
Two neurologists, a hospital site neurologist and a study investigator (L K or V R-C), confirmed the diagnosis of GBS according to the Brighton Collaboration definition (Box 1).2 Reviewers were blinded to vaccine history. Cases identified retrospectively were interviewed and additional information was collected from the patient’s medical record and the patient’s general practitioner. The Australian Childhood Immunisation Register was accessed to obtain immunisation information for children under 7 years of age.
The self-controlled case series (SCCS) method is an established method to assess vaccine safety9-12 (see the accompanying commentary by Hawken and Wilson [page 578]). It tests the null hypothesis that the incidence rate of events (incident GBS cases) is the same during a set period (risk period) after an exposure of interest (H1N1-containing vaccination) compared with the rate outside of this risk period. The method has the advantage of only requiring cases and of implicit control for confounding by non-time-varying variables.
As in previous studies, it was assumed that if vaccination was associated with an excess risk, GBS would present within 42 days.4,13 The background rate was adjusted for temporary delays in vaccination following GBS diagnosis by removing a 28-day prevaccination period. The analysis included the time-varying variables of trivalent and monovalent H1N1 vaccine, as well as month administered, and assumed that incidence followed a Poisson distribution conditional on the number of events that an individual experienced. We used standard and pseudolikelihood methods. The pseudolikelihood method was used to allow for the vaccine being contraindicated following the GBS diagnosis.14 The primary analysis included first episodes of confirmed cases (Brighton level 1–4), excluding those with incomplete immunisation history. Sensitivity analyses included: dropping the monthly adjustment; using 2-week risk periods (0–13, 14–27 and 28–42 days); including second episodes; restricting to cases of higher certainty (Brighton level 1–2); including cases not confirmed as GBS; and including cases without a complete immunisation history.
A sample size calculation was undertaken before the study.15 Assuming a 0–42-day risk “window” during an observation period of 1 year where 20% of the population were vaccinated, 60 participants were required to demonstrate an RI of 8 (as documented in 19763) with 90% power, allowing for 30% misclassification.
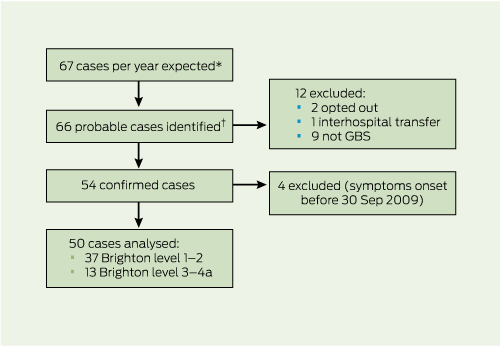
Sixty-six cases of probable GBS were identified in the 12-month study period (Box 2). Three cases were excluded, leaving 63 GBS episodes in 62 individuals. Of these, nine were excluded, as on review they did not meet diagnostic criteria for GBS. Four further confirmed cases were excluded, as the date of onset of symptoms predated the availability of the monovalent H1N1 vaccine. In six cases, data were only available from the hospital medical record (ie, neither the patient nor primary care physician were available). These cases with incomplete immunisation histories were excluded from the primary analysis, leaving 44 cases.
The GBS cases were in people aged 7–95 years (median, 48 years). The male to female ratio was 1.1:1. There were 54 confirmed GBS cases in the 12-month period at the 10 Victorian hospital sites. Based on the fact that these sites accounted for about 60% of GBS cases and the Victorian estimated resident population at 31 December 2009 was 5 496 000,16 the annual incidence of GBS was calculated to be 1.7 per 100 000 population.
Overall, 37 patients met Brighton level 1–2 and 13 patients met level 3–4a (Box 2). The second episode in the individual with two presentations was level 4a. Monovalent H1N1 vaccine had been received by 11 patients (eight Brighton level 1–2, two level 3–4a and one without GBS). A total of 12 patients had received TIV within the study period, or within 6 weeks of commencement of the study period (of these, seven were Brighton level 1–2, four were level 3–4a and one did not have GBS). Two individuals received both vaccines in the study period.
Three cases of GBS occurred within 42 days of receipt of monovalent H1N1 vaccine; one within each 2-week period following receipt of vaccine (at Days 9, 24 and 40; Box 3). Of these, two were Brighton level 1 and one was level 2. One case of GBS occurred within 42 days of seasonal TIV (at Day 27; Brighton level 4).
Following GBS diagnosis, two cases received seasonal TIV, but none received the monovalent H1N1 vaccine. The RI estimate from the primary analysis using the standard method was 3.41 (95% CI, 0.78–14.97), compared with the pseudolikelihood estimate of 3.17 (bootstrap 95% CI, 0–16.78). As the change was < 25%, the standard method was chosen as the primary analysis. SCCS sensitivity analyses produced an estimated non-significant RI rate of between 2 and 3 (Box 3). Following TIV, there was one case in the risk period and the RI was estimated as 0.69 (95% CI, 0.08–5.64). For TIV or monovalent H1N1 vaccine, there were four cases in the risk period and the RI was estimated at 1.71 (95% CI, 0.54–5.39).
Active surveillance in Australia for GBS following pandemic (H1N1) 2009 influenza A immunisation was crucial, given the increased risk found in the US in 1976.3 Our active GBS surveillance in Victoria was powered to detect a signal similar to the eightfold increase identified in that study. Our main analysis showed no evidence of a significantly increased risk of GBS following H1N1 immunisation. Three cases of GBS were identified within 42 days following administration of monovalent H1N1 vaccine, with an RI estimate of 3.41 (95% CI, 0.78–14.97).
Active GBS surveillance after pandemic H1N1/09 influenza immunisation was also undertaken in the US, using a number of different methodologies. The Vaccine Safety Datalink Project (2009–2010) used the self-controlled risk interval analysis, with the risk difference following monovalent influenza vaccine being five cases per million doses (95% CI, 0.5–9.5).17 Wise and colleagues, using active, population-based surveillance for incident GBS cases among 45 million people, observed a small increase, with 0.74 excess cases per million monovalent H1N1 vaccine doses (95% CI, 0.04–1.56).18 Yih and colleagues, using the Post-Licensure Rapid Immunization Safety Monitoring cohort active surveillance system, found an elevated but not statistically significant incidence rate ratio following receipt of monovalent H1N1 vaccine (2.5; 95% CI, 0.42–15.0).19 No statistically significant increase was identified in a European case–control series of monovalent H1N1 vaccine and GBS.20 Case recruitment varied considerably by region — there were 104 confirmed cases from five European countries with an adjusted odds ratio risk of GBS of 1.0 (95% CI, 0.3–2.7).20 A United Kingdom study assessed GBS risk following an AS03 adjuvanted H1N1 vaccine using an SCCS analysis of cases identified from electronic hospital episode data.21 It found that the RI was not significant (1.05; 95% CI, 0.37–2.24), with limited Brighton GBS case validation because of a low reporting rate from neurologists.21
Passive AEFI surveillance for conditions such as GBS has the potential limitation of poor case ascertainment. In China, a study detected 11 cases of GBS out of 89 million distributed doses of H1N1 vaccine.22 This compares with the National Vaccine Injury Compensation Program in Korea, which had 22 confirmed cases of GBS (Brighton level 1–3).23
A strength of our study was that it reviewed immunisation records from multiple sources, including hospital and general practitioner records, and included a detailed immunisation interview. By comprehensively reviewing admissions, neurophysiology test results and hospital International Classification of Diseases coding, we believe it is unlikely that any cases were missed at participating hospitals. Detailed review identified nine out of 63 cases (14.3%) that were not GBS according to the Brighton criteria. This is less than the 31% misclassification rate described elsewhere.13
A potential study limitation was the low rate of uptake for H1N1 vaccine, as higher population coverage rates increases the power to detect a difference in GBS incidence. Australia did not have an influenza immunisation register, but a representative survey of over 10 000 adults estimated that 18.9% of Australians had received monovalent H1N1 influenza vaccine by December 2009, which was close to our study power calculation of 20% coverage.24 A higher coverage rate (42.6%) was found in individuals aged 65 years and over who are routinely funded to receive the seasonal TIV. A second limitation of the study was the lack of complete immunisation history for six cases, with data only available from hospital records. Including these cases in the SCCS analysis did not significantly alter the relative incidence estimate. The study also had limited data on any infections or laboratory investigations before the GBS diagnosis. The logistics of the study were difficult, with ethics submissions and modifications required at each of the 10 study sites, highlighting the urgent requirement for multisite ethics committees in Australia for both interventional and non-interventional studies.25 This will be important for future AEFI research that needs to be established rapidly and, in these circumstances, ethics review should be expedited.
Our study was not powered to detect a small increase in GBS following H1N1 vaccination but was linked to the World Health Organization’s Global Vaccine Safety Initiative, which was powered to detect lower increases in the RI of GBS following H1N1 vaccination and to provide data on regional differences. A preliminary unpublished analysis of these data was recently presented and found an RI of GBS in the 42 days following H1N1 vaccination of 2.86 (95% CI, 1.87–4.34).26 This suggests that there was an increase in GBS cases following immunisation (of a similar magnitude estimated in our study), but that this increase is smaller than that described with the 1976 vaccine. This small increase in GBS risk must be considered in the context of the benefits of influenza vaccinations, including potential protection against severe influenza and GBS due to influenza infection.
In this Victorian study, we found that only a small proportion of GBS cases occurred following pandemic influenza immunisation, and that the RI of GBS following immunisation was not statistically different to the baseline rate. The study could not exclude smaller increases in the RI, which have been suggested in studies among larger populations with higher vaccine coverage18 and the preliminary analysis of an international meta-analysis of GBS monitoring studies.26 This highlights the role of international collaborations in active AEFI surveillance, to carefully assess the risks and benefits of population immunisation programs.
Received 26 March 2012, accepted 19 August 2012
- Nigel W Crawford1
- Allen Cheng2
- Nick Andrews3
- Patrick G Charles4
- Hazel J Clothier5
- Bruce Day2
- Timothy Day6
- Peter Gates7
- Richard Macdonell4
- Les Roberts8
- Victoria Rodriguez-Casero1
- Tissa Wijeratne9
- Lynette Kiers6
- 1 Royal Children’s Hospital, Melbourne, VIC.
- 2 Alfred Hospital and Monash University, Melbourne, VIC.
- 3 Health Protection Agency, London, UK.
- 4 Austin Health, Melbourne, VIC.
- 5 Murdoch Childrens Research Institute, Melbourne, VIC.
- 6 Royal Melbourne Hospital, Melbourne, VIC.
- 7 Barwon Health, Geelong, VIC.
- 8 St Vincent’s Hospital, Melbourne, VIC.
- 9 Department of Neurology, Western Hospital, Melbourne, VIC.
We thank Ainsley Swanson, Sherisse Celestino, Natalie Teasdale, Miranda Pellissier and Anne Lickliter for their help in data collection as the research assistants at the GBS active study sites.
This study was funded by CSL Ltd as part of postmarketing surveillance, with financial support for study nurses at each site. The funder did not have any role in data collection, analysis or the decision to publish.
- 1. Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent influenza A (H1N1) vaccine. N Engl J Med 2009; 361: 2405-2413.
- 2. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011; 29: 599-612.
- 3. Schonberger LB, Bergman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979; 110: 105-123.
- 4. Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166: 1301-1304.
- 5. Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database. Am J Epidemiol 2009; 169: 382-388.
- 6. Juurlink DN, Stukel TA, Kwong J, et al. Guillain-Barré syndrome after influenza vaccination in adults: a population-based study. Arch Intern Med 2006; 166: 2217-2221.
- 7. Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48: 48-56.
- 8. Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf 2009; 32: 309-323.
- 9. Andrews N, Miller E, Waight P, et al. Does oral polio vaccine cause intussusception in infants? Evidence from a sequence of three self-controlled cases series studies in the United Kingdom. Eur J Epidemiol 2001; 17: 701-706.
- 10. Stowe J, Kafatos G, Andrews N, Miller E. Idiopathic thrombocytopenic purpura and the second dose of MMR. Arch Dis Child 2008; 93: 182-183.
- 11. Farrington CP, Nash J, Miller E. Case series analysis of adverse reactions to vaccines: a comparative evaluation. Am J Epidemiol 1996; 143: 1165-1173.
- 12. Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 2009; 18: 7-26.
- 13. Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998; 339: 1797-1802.
- 14. Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics 2009; 10: 3-16.
- 15. Musonda P, Farrington CP, Whitaker HJ. Sample sizes for self-controlled case series studies. Stat Med 2006; 25: 2618-2631.
- 16. Australian Bureau of Statistics. State and regional indicators, Victoria, June 2010. (ABS Cat. No. 1367.2.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1367.2Jun+2010 (accessed May 2011).
- 17. Greene SK, Rett M, Weintraub ES, et al. Risk of confirmed Guillain-Barre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009-2010. Am J Epidemiol 2012; 175: 1100-1109.
- 18. Wise ME, Viray M, Sejvar JJ, et al. Guillain-Barre syndrome during the 2009-2010 H1N1 influenza vaccination campaign: population-based surveillance among 45 million Americans. Am J Epidemiol 2012; 175: 1110-1119.
- 19. Yih WK, Lee GM, Lieu TA, et al. Surveillance for adverse events following receipt of pandemic 2009 H1N1 vaccine in the Post-Licensure Rapid Immunization Safety Monitoring (PRISM) System, 2009-2010. Am J Epidemiol 2012; 175: 1120-1128.
- 20. Dieleman J, Romio S, Johansen K, et al; VAESCO-GBS Case-Control Study Group. Guillain-Barrre syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ 2011; 343: d3908.
- 21. Andrews N, Stowe J, Al-Shahi Salman R, Miller E. Guillain-Barré syndrome and H1N1 (2009) pandemic influenza vaccination using an AS03 adjuvanted vaccine in the United Kingdom: self-controlled case series. Vaccine 2011; 29: 7878-7882.
- 22. Liang X-F, Li L, Liu DW, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med 2011; 364: 638-647.
- 23. Choe YJ, Cho H, Bae GR, Lee JK. Guillain-Barré syndrome following receipt of influenza A (H1N1) 2009 monovalent vaccine in Korea with an emphasis on Brighton Collaboration case definition. Vaccine 2011; 29: 2066-2070.
- 24. Australian Institute of Health and Welfare. 2010 Pandemic Vaccination Survey: summary results. Canberra: AIHW, 2010. (AIHW Cat. No. PHE 128.) http://www.aihw.gov.au/publication-detail/?id=6442468387 (accessed Jan 2012).
- 25. Hicks SC, James RE, Wong N, et al; Australasian Gastro-Intestinal Trials Group. A case study evaluation of ethics review systems for multicentre clinical trials. Med J Aust 2009; 191: 280-282. <MJA full text>
- 26. Dodd CN, Romio SA, Izurieta H, et al. International collaborative case series safety monitoring for pandemic 2009 H1N1 vaccines: estimation of the risk of Guillain-Barré syndrome [abstract]. Proceedings of the 28th International Conference on Pharmacoepidemiology and Therapeutic Risk Management; 2012 Aug 23-26; Barcelona, Spain.





Abstract
Objectives: To determine the relative incidence (RI) of Guillain-Barré syndrome (GBS) in a single Australian state following pandemic (H1N1) 2009 influenza A immunisation (monovalent vaccine or seasonal trivalent influenza vaccine [TIV]) in 2009–2010.
Design, setting and participants: Active GBS surveillance (cases assessed by two neurologists according to the Brighton criteria) from 30 September 2009 to 30 September 2010, conducted at 10 hospitals in Victoria, Australia.
Main outcome measures: The RI of GBS in the risk window of 0–42 days after vaccination.
Results: Sixty-six potential GBS cases were identified, with complete data on 50 confirmed cases. The Victorian annual incidence of GBS was 1.7 per 100 000 population. Three cases had received monovalent vaccine and one case had received seasonal TIV within 42 days of symptom onset. The RI of GBS following monovalent vaccination was 3.4 (95% CI, 0.8–15.0). For TIV, there was one case in the risk period (RI, 0.69; 95% CI, 0.08–5.64).
Conclusions: This is the first published study reviewing GBS after a trivalent and/or monovalent influenza vaccine containing the pandemic (H1N1) 2009 strain, with only a small proportion of GBS cases occurring after influenza immunisation. H1N1-containing vaccines were not statistically associated with GBS, but this study could not exclude smaller increases in the RI. Active surveillance of adverse events following immunisation is required to maintain public and health care professional confidence in mass vaccine implementation programs.