Abbreviations
AGITG |
Australasian Gastro-Intestinal Trials Group |
HREC |
Human research ethics committee |
NEAF |
National ethics application form |
NHMRC |
National Health and Medical Research Council |
SSA |
Site-specific assessment |
Ethics review is an essential element of clinical research. In Australia, the review process is governed by the National statement on ethical conduct in human research.1 Until recently, ethics review and research governance approval for multicentre clinical trials in Australia were primarily conducted by human research ethics committees (HRECs) at individual sites. However, there has been ongoing concern that submitting identical applications to multiple HRECs is inefficient and creates delays in trial initiation, thus delaying patient recruitment and treatment.2-4 Concern has also existed around the expectation of HRECs to approve site governance, which they were not necessarily equipped to do.5,6
A new, centralised ethics review system was implemented in New South Wales in July 2007.7 Under the new system, central ethics approval is given by a lead HREC and covers all NSW public health sites listed in the submission. Once a trial is approved, each site is required to submit a separate site-specific assessment (SSA) of its trial governance capability. This covers the suitability of the research, the facilities required, staff expertise and regulatory considerations for the site.
The NSW centralised system coincided with updates to the national statement1 and the implementation of a national ethics application form (NEAF). These changes provided an opportunity to evaluate the time periods between trial submission and ethics and governance approval for two multicentre trials using different HREC review systems.
The times from trial submission to ethics and governance approval were obtained for sites involved in two international, multicentre clinical trials. The Australasian Gastro-Intestinal Trials Group (AGITG) was the sponsor of both investigator-initiated trials, which were coordinated through the National Health and Medical Research Council (NHMRC) Clinical Trials Centre (“the coordinating centre”) using standard operating procedures. Feedback on both review systems was obtained from site staff at the AGITG Annual Scientific Meeting study coordinator forum.
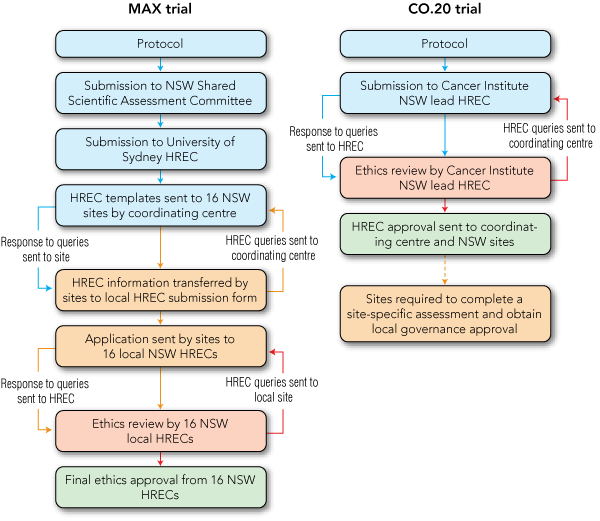
Both trials incorporated biological agents as palliative therapy for metastatic colorectal cancer. The MAX trial is a locally developed randomised controlled phase II–III study of mitomycin C, capecitabine and bevacizumab. From June 2005 to July 2007, it recruited 471 patients from 43 sites (38 in Australia, two in New Zealand and three in the United Kingdom).8 Ethics approval for the MAX trial was obtained via the non-centralised system (Box 1). Overall scientific and ethics approval of the study was required from the NSW Shared Scientific Assessment Committee and the coordinating centre’s local HREC at the University of Sydney. The protocol was then submitted to each site, which then conveyed the information to its local HREC via site-specific documentation.
The CO.20 trial is a randomised controlled phase III study of brivanib and cetuximab compared with cetuximab and placebo, developed by the National Cancer Institute of Canada in collaboration with the AGITG.9 The study opened in March 2008 in Australia, New Zealand and Singapore. About 50 sites will recruit 370 patients over 3 years. The CO.20 protocol and NEAF were submitted by the coordinating centre to the lead HREC of the Cancer Institute NSW using the centralised system (Box 1). Once HREC approval had been obtained, site staff submitted an online SSA to their local research governance office.
Within Australia, 26 of the study sites were involved in both trials. The MAX trial was conducted in three regulatory regions, requiring 41 ethics applications: one to the HREC of the coordinating centre, 38 to Australian site HRECs (16 in NSW), and one regional application each to New Zealand and the UK. At the time of our review, 35 of the 50 potential CO.20 sites had completed the ethics approval process, 16 using centralised review with the NEAF (14 in NSW, one in Victoria and one in Queensland) and 19 being reviewed by individual site HRECs (four sites using the NEAF and 15 using site-specific documentation). The median times taken for ethics and governance approval for both trials are listed in Box 2.
Ethical issues raised in relation to both studies included protocol interpretation, clarification of methods, safety analyses, statistics, confidentiality concerns, tissue banking, grammatical changes to consent forms, and the provision of interpreters for quality-of-life questionnaires.
The median time taken to obtain ethics approval was 23 days shorter for the CO.20 trial (77 days) than for the MAX trial (100 days). However, the additional requirement to submit an SSA for governance approval at CO.20 sites meant that the time taken to obtain overall study approval was 37 days longer for the CO.20 trial than for the MAX trial (Box 2). Delays in obtaining SSA approval related to the lack of a dedicated full-time governance officer at sites and variations in site research governance processes.
As the coordinating centre did not have direct contact with the site HRECs for the MAX trial, site staff collated and relayed queries and responses relating to ethical issues. In contrast, HREC queries for the CO.20 trial were dealt with directly by the coordinating centre, which helped facilitate the review process. Overall, both the coordinating centre and site staff found the centralised approach to be more efficient than the non-centralised approach, with the time spent in collation, submission and correspondence being greatly reduced. Processes were also improved by the ability to maintain standardised documentation at the coordinating centre for all of the centrally incorporated sites.
The introduction of centralised ethics review for multicentre research in Australia has been awaited by many researchers and collaborative trials groups. However, evaluation of different review systems has been limited. In our study, ethics approval for the two trials was obtained through completely independent systems that are not directly comparable. Nevertheless, it was clear that any difference in time to approval was outweighed by the overall time taken.
The fact that it took 77 days to obtain HREC approval using the new centralised system was mainly due to repeated revision of the protocol by the lead HREC. Additional time was then required for research governance approval at multiple sites. The centralised system had only recently been introduced at the time of our study, and familiarisation with the new processes may have improved since then. However, we have not noticed any significant change in approval times for our current work. The results of our study may not be applicable to other types of studies, but they highlight the need for further evaluation of approval processes.
The difficulties encountered in our review of multicentre research are not unique to Australia.10-12 Both NZ and the UK have introduced a dual system that incorporates reviews by both local and multiregion HRECs. These systems appear to have reduced the burden of multisite ethics review, with an estimated 30% reduction in reviews in the UK and a decrease in the complaints and issues encountered by researchers.7
Currently, no formal agreement exists between NSW and other Australian states on reciprocal acceptance of central ethics approval. However, in 2007, the federal government funded the Harmonisation of Multi-centre Ethical Review project to develop a national system of scientific and ethics review that will allow a review by any HREC in any jurisdiction to be recognised by all jurisdictions. For proponents of a centralised system, this may be seen as a positive move forward, as a national overhaul of our research ethics system is needed to instigate notable change. The exact way to achieve this is still under consultation.
The introduction of a centralised ethics review process in NSW did not reduce the overall time taken for trial approval, but was beneficial in reducing the time and effort spent on duplicating administrative procedures and in promoting consistency of site documentation. For Australia to continue to conduct high-quality clinical research, it is imperative that we improve the ethics review system to expedite ethics approval for trials that may allow patients earlier access to potential therapies.
1 Ethics approval process in New South Wales using the non-centralised review system (for the MAX trial8) and the centralised review system (for the CO.20 trial9)
 | |||||||||||||||
|
HREC = human research ethics committee. | |||||||||||||||
2 Median time taken for ethics and governance approval at Australian sites for the MAX8 and CO.209 clinical trials
|
MAX trial |
CO.20 trial |
|||||||||||||
Type of approval |
Number of sites |
Median time in days (range) |
Number of sites |
Median time in days (range) |
|||||||||||
Approval by HREC (Australian sites) |
38* |
100 (14–394) |
19† |
89 (20–139) |
|||||||||||
Approval by HREC (NSW sites only) |
16 |
100 (36–161) |
14 |
77 |
|||||||||||
Site-specific assessment of trial governance capability |
na |
na |
14 |
60 (20–79) |
|||||||||||
HREC = human research ethics committee. na = not applicable. NSW = New South Wales. * Includes NSW sites. † Excludes NSW sites. |
|||||||||||||||
Received 21 January 2009, accepted 15 June 2009
- Sian C Hicks1
- Rebecca E James2
- Nicole Wong2
- Niall C Tebbutt3
- Kate Wilson2
- 1 Integrated Cancer Research Group, Prince of Wales Clinical School, University of New South Wales, Sydney, NSW.
- 2 NHMRC Clinical Trials Centre, University of Sydney, Sydney, NSW.
- 3 Austin Health, Melbourne, VIC.
We would like to thank Rhana Pike (NHMRC Clinical Trials Centre) for assistance with the manuscript.
None identified.
- 1. National Health and Medical Research Council. National statement on ethical conduct in human research. Canberra: NHMRC, 2007. http://www.nhmrc.gov.au/PUBLICATIONS/synopses/_files/e72.pdf (accessed Jul 2009).
- 2. Fox RM. Debate: should Australia move towards a centralized ethics committees system? The case for. Intern Med J 2005; 35: 247-248.
- 3. Roberts LM, Bowyer L, Homer CS, Brown MA. Multicentre research: negotiating the ethics approval obstacle course [letter]. Med J Aust 2004; 180: 139. <MJA full text>
- 4. Whiteman DC, Webb PM, Purdie DM, Green AC. National ethics committee urgently needed. Med J Aust 2003; 178: 187. <MJA full text>
- 5. Christie DRH, Gabriel GS, Dear K. Adverse effects of a multicentre system for ethics approval on the progress of a prospective multicentre trial of cancer treatment: how many patients die waiting? Intern Med J 2007; 37: 680-686.
- 6. Poustie SJ, Taylor DM, Forbes AB, et al. Implementing a research governance framework for clinical and public health research. Med J Aust 2006; 185: 623-626. <MJA full text>
- 7. New South Wales Department of Health. Research — authorisation of proposals to conduct research on humans within NSW public health system. Policy directive PD2007_043, 18 July 2007, file no. 05/1874-4. http://www.health. nsw.gov.au/policies/pd/2007/PD2007_043.html (accessed Aug 2009).
- 8. ClinicalTrials.gov (US National Institutes of Health). The MAX Study: a randomised phase II/III study to evaluate the role of mitomycin C, avastin and xeloda in patients with untreated metastatic colorectal cancer. (ClinicalTrials.gov identifier NCT00294359; Australian and New Zealand Clinical Trials Registry No. ACTRN012605000025639.) http://clinicaltrials.gov/ct2/show/NCT00294359 (accessed Aug 2009).
- 9. ClinicalTrials.gov (US National Institutes of Health). A phase III randomized study of brivanib alaninate (BMS-582664) in combination with cetuximab (Erbitux) versus placebo in combination with cetuximab (Erbitux) in patients previously treated with combination chemotherapy for metastatic colorectal carcinoma. (ClinicalTrials.gov identifier NCT00640471; Australian and New Zealand Clinical Trials Registry No. ACTRN12607000589482.) http://clinicaltrials.gov/ct2/show/NCT00640471 (accessed Aug 2009).
- 10. Fitzgerald MH, Phillips PA. Centralized and non-centralized ethics review: a five nation study. Account Res 2006; 13: 47-74.
- 11. Chaddah MR. The Ontario Cancer Research Ethics Board: a central REB that works. Curr Oncol 2008; 15: 49-52.
- 12. Christian MC, Goldberg JL, Killen J, et al. A central institutional review board for multi-institutional trials. N Engl J Med 2002; 346: 1405-1408.





Abstract
Objective: To evaluate the difference in time taken for ethics and site governance approval for multicentre clinical trials using two different systems of ethics review.
Design: We evaluated the times to final ethics and governance approval for two international, multicentre clinical trials of treatment for metastatic colorectal cancer: the MAX trial, using a non-centralised ethics review system, and the CO.20 trial, using the new New South Wales centralised ethics review system.
Main outcome measure: Time from trial submission to overall study approval.
Results: The median time taken to obtain ethics approval for the MAX trial at 16 NSW sites was 100 days (range, 36–161 days). The median time to obtain central ethics approval for the CO.20 trial at 14 NSW sites was 77 days, with an additional 60 days (range 20–79 days) required to obtain site-specific research governance approval.
Conclusions: Any difference in time to approval between the review systems was outweighed by the overall time taken. However, the time spent by both the coordinating centre and local sites in collation, submission and correspondence was greatly reduced, and the centralised process allowed for standardised documentation at all study sites.